Improved Serial Sectioning Techniques for Correlative Light-Electron Microscopy Mapping of Human Langerhans Islets
- PMID: 29622846
- PMCID: PMC5880804
- DOI: 10.1267/ahc.17020
Improved Serial Sectioning Techniques for Correlative Light-Electron Microscopy Mapping of Human Langerhans Islets
Abstract
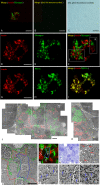
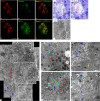
Combined analysis of immunostaining for various biological molecules coupled with investigations of ultrastructural features of individual cells is a powerful approach for studies of cellular functions in normal and pathological conditions. However, weak antigenicity of tissues fixed by conventional methods poses a problem for immunoassays. This study introduces a method of correlative light and electron microscopy imaging of the same endocrine cells of compact and diffuse islets from human pancreatic tissue specimens. The method utilizes serial sections obtained from Epon-embedded specimens fixed with glutaraldehyde and osmium tetroxide. Double-immunofluorescence staining of thick Epon sections for endocrine hormones (insulin and glucagon) and regenerating islet-derived gene 1 α (REG1α) was performed following the removal of Epoxy resin with sodium ethoxide, antigen retrieval by autoclaving, and de-osmification treatment with hydrogen peroxide. The immunofluorescence images of endocrine cells were superimposed with the electron microscopy images of the same cells obtained from serial ultrathin sections. Immunofluorescence images showed well-preserved secretory granules in endocrine cells, whereas electron microscopy observations demonstrated corresponding secretory granules and intracellular organelles in the same cells. In conclusion, the correlative imaging approach developed by us may be useful for examining ultrastructural features in combination with immunolocalisation of endocrine hormones in the same human pancreatic islets.
Keywords: Epon-embedded; compact and diffuse islets; correlative microscopy; hormone; immunostaining.
Figures





Similar articles
-
Osmium-Resistant Fluorescent Proteins and In-Resin Correlative Light-Electron Microscopy of Epon-Embedded Mammalian Cultured Cells.Methods Mol Biol. 2023;2564:287-297. doi: 10.1007/978-1-0716-2667-2_15. Methods Mol Biol. 2023. PMID: 36107349
-
Heat-induced Antigen Retrieval in Conventionally Processed Epon-embedded Specimens: Procedures and Mechanisms.J Histochem Cytochem. 2014 Aug;62(8):584-97. doi: 10.1369/0022155414537899. Epub 2014 May 21. J Histochem Cytochem. 2014. PMID: 24850662
-
In-resin CLEM of Epon-embedded cells using proximity labeling.Sci Rep. 2022 Jul 1;12(1):11130. doi: 10.1038/s41598-022-15438-6. Sci Rep. 2022. PMID: 35778550 Free PMC article.
-
Recent advances in in-resin correlative light and electron microscopy of Epon-embedded cells.Microscopy (Oxf). 2023 Oct 9;72(5):383-387. doi: 10.1093/jmicro/dfad028. Microscopy (Oxf). 2023. PMID: 37217182
-
Correlative microscopy.Arch Biochem Biophys. 2015 Sep 1;581:98-110. doi: 10.1016/j.abb.2015.05.017. Epub 2015 Jun 10. Arch Biochem Biophys. 2015. PMID: 26072116 Review.
Cited by
-
Three-dimensional structural analysis of mitochondria composing each subtype of fast-twitch muscle fibers in chicken.J Vet Med Sci. 2022 Jun 17;84(6):809-816. doi: 10.1292/jvms.22-0080. Epub 2022 Apr 14. J Vet Med Sci. 2022. PMID: 35418525 Free PMC article.
-
Sub-chronic toxicity of Garcinia atroviridis Griff Fruit's ethanol extract on Wistar rats (Ratus norvegicus).J Adv Pharm Technol Res. 2019 Oct-Dec;10(4):178-183. doi: 10.4103/japtr.JAPTR_70_19. J Adv Pharm Technol Res. 2019. PMID: 31742118 Free PMC article.
-
Methods for array tomography with correlative light and electron microscopy.Med Mol Morphol. 2019 Mar;52(1):8-14. doi: 10.1007/s00795-018-0194-y. Epub 2018 May 31. Med Mol Morphol. 2019. PMID: 29855715 Review.
-
Advanced Imaging Techniques for the Characterization of Subcellular Organelle Structure in Pancreatic Islet β Cells.Compr Physiol. 2023 Dec 29;14(1):5243-5267. doi: 10.1002/cphy.c230002. Compr Physiol. 2023. PMID: 38158370 Free PMC article.
-
Hydroxynonenal causes Langerhans cell degeneration in the pancreas of Japanese macaque monkeys.PLoS One. 2021 Nov 8;16(11):e0245702. doi: 10.1371/journal.pone.0245702. eCollection 2021. PLoS One. 2021. PMID: 34748564 Free PMC article.
References
-
- Aida K., Saitoh S., Nishida Y., Yokota S., Ohno S., Mao X., Akiyama D., Tanaka S., Awata T., Shimada A., Oikawa Y., Shimura H., Furuya F., Takizawa S., Ichijo M., Ichijo S., Itakura J., Fujii H., Hashiguchi A., Takasawa S., Endo T. and Kobayashi T. (2014) Distinct cell clusters touching islet cells induce islet cell replication in association with over-expression of Regenerating Gene (REG) protein in fulminant type 1 diabetes. PLoS One 9; e95110. - PMC - PubMed
-
- Baskin D. G., Erlandsen S. L. and Parsons J. A. (1979) Immunocytochemistry with osmium-feed tissue. 1. Light microscopic localization of growth hormone and prolactin with the unlabeled antibody-enzyme method. J. Histochem. Cytochem. 27; 867–872. - PubMed
-
- Bendayan M. and Zollinger M. (1983) Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J. Histochem. Cytochem. 31; 101–109. - PubMed
-
- Ben-Othman N., Vieira A., Courtney M., Record F., Gjernes E., Avolio F., Hadzic B., Druelle N., Napolitano T., Navarro-Sanz S., Silvano S., Al-Hasani K., Pfeifer A., Lacas-Gervais S., Leuckx G., Marroquí L., Thévenet J., Madsen O. D., Eizirik D. L., Heimberg H., Kerr-Conte J., Pattou F., Mansouri A. and Collombat P. (2017) Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 168; 73–85. - PubMed
-
- Bensley R. R. (1911) Studies on the pancreas of the guinea pig. Am. J. Anat. 12; 297–388.
LinkOut - more resources
Full Text Sources
Other Literature Sources

