Enzyme-free genetic copying of DNA and RNA sequences
- PMID: 29623122
- PMCID: PMC5870163
- DOI: 10.3762/bjoc.14.47
Enzyme-free genetic copying of DNA and RNA sequences
Abstract
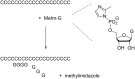
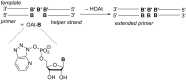
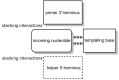
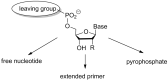
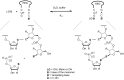
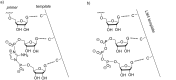
The copying of short DNA or RNA sequences in the absence of enzymes is a fascinating reaction that has been studied in the context of prebiotic chemistry. It involves the incorporation of nucleotides at the terminus of a primer and is directed by base pairing. The reaction occurs in aqueous medium and leads to phosphodiester formation after attack of a nucleophilic group of the primer. Two aspects of this reaction will be discussed in this review. One is the activation of the phosphate that drives what is otherwise an endergonic reaction. The other is the improved mechanistic understanding of enzyme-free primer extension that has led to a quantitative kinetic model predicting the yield of the reaction over the time course of an assay. For a successful modeling of the reaction, the strength of the template effect, the inhibitory effect of spent monomers, and the rate constants of the chemical steps have to be determined experimentally. While challenges remain for the high fidelity copying of long stretches of DNA or RNA, the available data suggest that enzyme-free primer extension is a more powerful reaction than previously thought.
Keywords: DNA; RNA; base pairing; enzyme-free primer extension; nucleotides; oligonucleotides; replication.
Figures

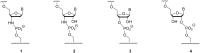
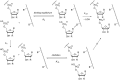



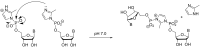

References
-
- Kornberg A, Baker T A. DNA Replication. 2nd ed. Mill Valley: University Science Books; 2005.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
