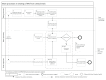
Classification of processes involved in sharing individual participant data from clinical trials
- PMID: 29623192
- PMCID: PMC5861517
- DOI: 10.12688/f1000research.13789.2
Classification of processes involved in sharing individual participant data from clinical trials
Abstract
Background: In recent years, a cultural change in the handling of research data has resulted in the promotion of a culture of openness and an increased sharing of data. In the area of clinical trials, sharing of individual participant data involves a complex set of processes and the interaction of many actors and actions. Individual services and tools to support data sharing are becoming available, but what is missing is a detailed, structured and comprehensive list of processes and subprocesses involved and the tools and services needed. Methods: Principles and recommendations from a published consensus document on data sharing were analysed in detail by a small expert group. Processes and subprocesses involved in data sharing were identified and linked to actors and possible supporting services and tools. Definitions adapted from the business process model and notation (BPMN) were applied in the analysis. Results: A detailed and comprehensive tabulation of individual processes and subprocesses involved in data sharing, structured according to 9 main processes, is provided. Possible tools and services to support these processes are identified and grouped according to the major type of support. Conclusions: The identification of the individual processes and subprocesses and supporting tools and services, is a first step towards development of a generic framework or architecture for the sharing of data from clinical trials. Such a framework is needed to provide an overview of how the various actors, research processes and services could interact to form a sustainable system for data sharing.
Keywords: business process model; clinical trial; data sharing; generic framework; individual participant data (IPD); process.
Conflict of interest statement
No competing interests were disclosed.
Figures
References
-
- Skoog M, Saarimäki JM, Gluud C, et al. : Report on Transparency and Registration in Clinical Research in the Nordic countries. Nordic Trial Alliance Working Group 6 on Transparency and Registration.2015; accessed 15/01/2018. Reference Source
-
- Tudur Smith C, Hopkins C, Sydes M, et al. : Good Practice Principles for Sharing Individual Participant Data from Publicly Funded Clinical Trials.2015; accessed 15/01/2018. Reference Source
-
- ANDS guide: Publishing and sharing sensitive data. Australian National Data Service.2017; accessed 15/01/2018. Reference Source
LinkOut - more resources
Full Text Sources
Other Literature Sources