Enzymatic Processes to Unlock the Lignin Value
- PMID: 29623274
- PMCID: PMC5874288
- DOI: 10.3389/fbioe.2018.00020
Enzymatic Processes to Unlock the Lignin Value
Abstract
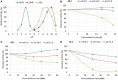
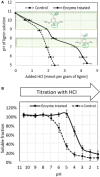
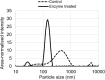
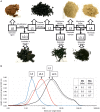
Main hurdles of lignin valorization are its diverse chemical composition, recalcitrance, and poor solubility due to high-molecular weight and branched structure. Controlled fragmentation of lignin could lead to its use in higher value products such as binders, coatings, fillers, etc. Oxidative enzymes (i.e., laccases and peroxidases) have long been proposed as a potentially promising tool in lignin depolymerization. However, their application was limited to ambient pH, where lignin is poorly soluble in water. A Finnish biotechnology company, MetGen Oy, that designs and supplies industrial enzymes, has developed and brought to market several lignin oxidizing enzymes, including an extremely alkaline lignin oxidase MetZyme® LIGNO™, a genetically engineered laccase of bacterial origin. This enzyme can function at pH values as high as 10-11 and at elevated temperatures, addressing lignin at its soluble state. In this article, main characteristics of this enzyme as well as its action on bulk lignin coming from an industrial process are demonstrated. Lignin modification by MetZyme® LIGNO™ was characterized by size exclusion chromatography, UV spectroscopy, and dynamic light scattering for monitoring particle size of solubilized lignin. Under highly alkaline conditions, laccase treatment not only decreased molecular weight of lignin but also increased its solubility in water and altered its dispersion properties. Importantly, organic solvent-free soluble lignin fragmentation allowed for robust industrially relevant membrane separation technologies to be applicable for product fractionation. These enzyme-based solutions open new opportunities for biorefinery lignin valorization thus paving the way for economically viable biorefinery business.
Keywords: biomass; enzyme; laccase; lignin; lignocellulosic; oxidoreductase.
Figures

References
-
- Boyd D. B. (1985). Leaving group ability and pKa, in elimination reactions. J. Org. Chem. 50, 885–886.10.1021/jo00206a033 - DOI
-
- Essery M. (2017). First Higher-Value Chemical Derived from Lignin to Hit Market in 2021. Lux Research; Available at: http://www.luxresearchinc.com/news-and-events/press-releases/read/first-...
-
- Frost and Sullivan. (2014). High-Value Opportunities for Lignin: Ready for Liftoff. Available at: http://www.frost.com/sublib/display-market-insight.do?id=290584392
LinkOut - more resources
Full Text Sources
Other Literature Sources

