Incorporating PROMIS Symptom Measures into Primary Care Practice-a Randomized Clinical Trial
- PMID: 29623512
- PMCID: PMC6082211
- DOI: 10.1007/s11606-018-4391-0
Incorporating PROMIS Symptom Measures into Primary Care Practice-a Randomized Clinical Trial
Abstract
Background: Symptoms account for more than 400 million clinic visits annually in the USA. The SPADE symptoms (sleep, pain, anxiety, depression, and low energy/fatigue) are particularly prevalent and undertreated.
Objective: To assess the effectiveness of providing PROMIS (Patient-Reported Outcome Measure Information System) symptom scores to clinicians on symptom outcomes.
Design: Randomized clinical trial conducted from March 2015 through May 2016 in general internal medicine and family practice clinics in an academic healthcare system.
Participants: Primary care patients who screened positive for at least one SPADE symptom.
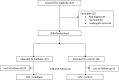
Interventions: After completing the PROMIS symptom measures electronically immediately prior to their visit, the 300 study participants were randomized to a feedback group in which their clinician received a visual display of symptom scores or a control group in which scores were not provided to clinicians.
Main measures: The primary outcome was the 3-month change in composite SPADE score. Secondary outcomes were individual symptom scores, symptom documentation in the clinic note, symptom-specific clinician actions, and patient satisfaction.
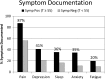
Key results: Most patients (84%) had multiple clinically significant (T-score ≥ 55) SPADE symptoms. Both groups demonstrated moderate symptom improvement with a non-significant trend favoring the feedback compared to control group (between-group difference in composite T-score improvement, 1.1; P = 0.17). Symptoms present at baseline resolved at 3-month follow-up only one third of the time, and patients frequently still desired treatment. Except for pain, clinically significant symptoms were documented less than half the time. Neither symptom documentation, symptom-specific clinician actions, nor patient satisfaction differed between treatment arms. Predictors of greater symptom improvement included female sex, black race, fewer medical conditions, and receiving care in a family medicine clinic.
Conclusions: Simple feedback of symptom scores to primary care clinicians in the absence of additional systems support or incentives is not superior to usual care in improving symptom outcomes.
Trial registration: clinicaltrials.gov identifier: NCT02383862.
Keywords: clinical trial; feedback; patient-reported outcomes; primary care; symptoms.
Conflict of interest statement
The authors declare that they do not have a conflict of interest.
Figures
Comment in
-
Use of PROs in Primary Care: PROMIS or Disappointment?J Gen Intern Med. 2018 Aug;33(8):1207-1208. doi: 10.1007/s11606-018-4511-x. J Gen Intern Med. 2018. PMID: 29872957 Free PMC article. No abstract available.
Similar articles
-
A qualitative study of patients' perceptions of the utility of patient-reported outcome measures of symptoms in primary care clinics.Qual Life Res. 2018 Dec;27(12):3157-3166. doi: 10.1007/s11136-018-1968-3. Epub 2018 Aug 14. Qual Life Res. 2018. PMID: 30109471
-
Does Sharing Patient-Reported Outcomes with Doctors Improve Patient Symptoms? [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2018 May. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2018 May. PMID: 37099662 Free Books & Documents. Review.
-
PROMIS 4-item measures and numeric rating scales efficiently assess SPADE symptoms compared with legacy measures.J Clin Epidemiol. 2019 Nov;115:116-124. doi: 10.1016/j.jclinepi.2019.06.018. Epub 2019 Jul 19. J Clin Epidemiol. 2019. PMID: 31330252 Clinical Trial.
-
The SPADE Symptom Cluster in Primary Care Patients With Chronic Pain.Clin J Pain. 2016 May;32(5):388-93. doi: 10.1097/AJP.0000000000000286. Clin J Pain. 2016. PMID: 26295379 Free PMC article. Clinical Trial.
-
Distinct Wound Healing and Quality-of-Life Outcomes in Subgroups of Patients With Venous Leg Ulcers With Different Symptom Cluster Experiences.J Pain Symptom Manage. 2017 May;53(5):871-879. doi: 10.1016/j.jpainsymman.2016.12.336. Epub 2017 Jan 4. J Pain Symptom Manage. 2017. PMID: 28063868 Review.
Cited by
-
Patient Reported Outcomes and Unscheduled Health Services use During Oral Anti-Cancer Treatment.J Pain Symptom Manage. 2023 Feb;65(2):e115-e121. doi: 10.1016/j.jpainsymman.2022.10.003. Epub 2022 Oct 14. J Pain Symptom Manage. 2023. PMID: 36244640 Free PMC article.
-
Generalized anxiety disorder screening scores are associated with greater treatment need among Veterans with depression.J Psychiatr Res. 2024 Sep;177:31-38. doi: 10.1016/j.jpsychires.2024.07.003. Epub 2024 Jul 3. J Psychiatr Res. 2024. PMID: 38971054 Free PMC article.
-
A qualitative study of patients' perceptions of the utility of patient-reported outcome measures of symptoms in primary care clinics.Qual Life Res. 2018 Dec;27(12):3157-3166. doi: 10.1007/s11136-018-1968-3. Epub 2018 Aug 14. Qual Life Res. 2018. PMID: 30109471
-
Adoption of Patient-Reported Outcomes by Health Systems and Physician Practices in the USA.J Gen Intern Med. 2022 Nov;37(15):3885-3892. doi: 10.1007/s11606-022-07631-0. Epub 2022 Apr 28. J Gen Intern Med. 2022. PMID: 35484368 Free PMC article.
-
Exploring the efficacy and safety of a novel standardized ashwagandha (Withania somnifera) root extract (Witholytin®) in adults experiencing high stress and fatigue in a randomized, double-blind, placebo-controlled trial.J Psychopharmacol. 2023 Nov;37(11):1091-1104. doi: 10.1177/02698811231200023. Epub 2023 Sep 23. J Psychopharmacol. 2023. PMID: 37740662 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical