The Potential Clinical and Economic Value of Primary Tumour Identification in Metastatic Cancer of Unknown Primary Tumour: A Population-Based Retrospective Matched Cohort Study
- PMID: 29623630
- PMCID: PMC6103931
- DOI: 10.1007/s41669-017-0051-2
The Potential Clinical and Economic Value of Primary Tumour Identification in Metastatic Cancer of Unknown Primary Tumour: A Population-Based Retrospective Matched Cohort Study
Abstract
Purpose: Several genomic tests have recently been developed to identify the primary tumour in cancer of unknown primary tumour (CUP). However, the value of identifying the primary tumour in clinical practice for CUP patients remains questionable and difficult to prove in randomized trials.
Objective: We aimed to assess the clinical and economic value of primary tumour identification in CUP using a retrospective matched cohort study.
Methods: We used the Manitoba Cancer Registry to identify all patients initially diagnosed with metastatic cancer between 2002 and 2011. We defined patients as having CUP if their primary tumour was found 6 months or more after initial diagnosis or never found during the course of disease. Otherwise, we considered patients to have metastatic cancer from a known primary tumour (CKP). We linked all patients with Manitoba Health databases to estimate their direct healthcare costs using a phase-of-care approach. We used the propensity score matching technique to match each CUP patient with a CKP patient on clinicopathologic characteristics. We compared treatment patterns, overall survival (OS) and phase-specific healthcare costs between the two patient groups and assessed association with OS using Cox regression adjustment.
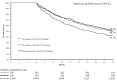
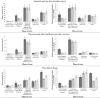
Results: Of 5839 patients diagnosed with metastatic cancer, 395 had CUP (6.8%); 1:1 matching created a matched group of 395 CKP patients. CUP patients were less likely to receive surgery, radiation, hormonal and targeted therapy and more likely to receive cytotoxic empiric chemotherapeutic agents. Having CUP was associated with reduced OS (hazard ratio [HR] 1.31; 95% confidence interval 1.1-1.58), but this lost statistical significance with adjustment for treatment differences. CUP patients had a significant increase in the mean net cost of initial diagnostic workup before diagnosis and a significant reduction in the mean net cost of continuing cancer care.
Conclusion: Identifying the primary tumour in CUP patients might enable the use of more effective therapies, improve OS and allow more efficient allocation of healthcare resources.
Conflict of interest statement
MBH, EW, SMM, MB, GR, SS, PKR, JSH and GSZ declare no potential conflicts of interest.
Figures

References
-
- BC Cancer Agency. Cancer management guidelines. 2016. http://www.bccancer.bc.ca/HPI/CancerManagementGuidelines/default.htm. Accessed 18 Aug 2016.
-
- Briasoulis E, Pavlidis N. Cancer of unknown primary origin. Oncologist. 1997;2(3):142–152. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

