Pseudotyping of HIV-1 with Human T-Lymphotropic Virus 1 (HTLV-1) Envelope Glycoprotein during HIV-1-HTLV-1 Coinfection Facilitates Direct HIV-1 Infection of Female Genital Epithelial Cells: Implications for Sexual Transmission of HIV-1
- PMID: 29624497
- PMCID: PMC5885023
- DOI: 10.1128/mSphere.00038-18
Pseudotyping of HIV-1 with Human T-Lymphotropic Virus 1 (HTLV-1) Envelope Glycoprotein during HIV-1-HTLV-1 Coinfection Facilitates Direct HIV-1 Infection of Female Genital Epithelial Cells: Implications for Sexual Transmission of HIV-1
Abstract
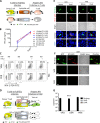
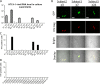
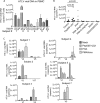
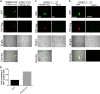
Female genital epithelial cells cover the genital tract and provide the first line of protection against infection with sexually transmitted pathogenic viruses. These cells normally are impervious to HIV-1. We report that coinfection of cells by HIV-1 and another sexually transmitted virus, human T-lymphotropic virus 1 (HTLV-1), led to production of HIV-1 that had expanded cell tropism and was able to directly infect primary vaginal and cervical epithelial cells. HIV-1 infection of epithelial cells was blocked by neutralizing antibodies against the HTLV-1 envelope (Env) protein, indicating that the infection was mediated through HTLV-1 Env pseudotyping of HIV-1. Active replication of HIV-1 in epithelial cells was demonstrated by inhibition with anti-HIV-1 drugs. We demonstrated that HIV-1 derived from peripheral blood of HIV-1-HTLV-1-coinfected subjects could infect primary epithelial cells in an HTLV-1 Env-dependent manner. HIV-1 from subjects infected with HIV-1 alone was not able to infect epithelial cells. These results indicate that pseudotyping of HIV-1 with HTLV-1 Env can occur in vivo Our data further reveal that active replication of both HTLV-1 and HIV-1 is required for production of pseudotyped HIV-1. Our findings indicate that pseudotyping of HIV-1 with HTLV-1 Env in coinfected cells enabled HIV-1 to directly infect nonpermissive female genital epithelial cells. This phenomenon may represent a risk factor for enhanced sexual transmission of HIV-1 in regions where virus coinfection is common.IMPORTANCE Young women in certain regions of the world are at very high risk of acquiring HIV-1, and there is an urgent need to identify the factors that promote HIV-1 transmission. HIV-1 infection is frequently accompanied by infection with other pathogenic viruses. We demonstrate that coinfection of cells by HIV-1 and HTLV-1 can lead to production of HIV-1 pseudotyped with HTLV-1 Env that is able to directly infect female genital epithelial cells both in vitro and ex vivo Given the function of these epithelial cells as genital mucosal barriers to pathogenic virus transmission, the ability of HIV-1 pseudotyped with HTLV-1 Env to directly infect female genital epithelial cells represents a possible factor for increased risk of sexual transmission of HIV-1. This mechanism could be especially impactful in settings such as Sub-Saharan Africa and South America, where HIV-1 and HTLV-1 are both highly prevalent.
Keywords: envelope glycoprotein; epithelial cells; human T-cell leukemia virus; human immunodeficiency virus; primary T-cells; pseudotype; retroviruses; sexual transmission; virus tropism.
Copyright © 2018 Tang et al.
Figures
References
-
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Li X, vanCott T, Quinn TC, Rakai Project Team . 2001. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI103397/AI/NIAID NIH HHS/United States
- U01 AI031834/AI/NIAID NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- U54 MD007586/MD/NIMHD NIH HHS/United States
- DP1 AI106275/AI/NIAID NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U01 AI103401/AI/NIAID NIH HHS/United States
- R25 GM059994/GM/NIGMS NIH HHS/United States
- U01 AI103390/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 AI103408/AI/NIAID NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
