Pharmacokinetics of efavirenz in patients on antituberculosis treatment in high human immunodeficiency virus and tuberculosis burden countries: A systematic review
- PMID: 29624706
- PMCID: PMC6046471
- DOI: 10.1111/bcp.13600
Pharmacokinetics of efavirenz in patients on antituberculosis treatment in high human immunodeficiency virus and tuberculosis burden countries: A systematic review
Abstract
Aims: Efavirenz (EFV) and rifampicin-isoniazid (RH) are cornerstone drugs in human immunodeficiency virus (HIV)-tuberculosis (TB) coinfection treatment but with complex drug interactions, efficacy and safety challenges. We reviewed recent data on EFV and RH interaction in TB/HIV high-burden countries.
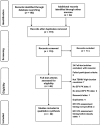
Methods: We conducted a systematic review of studies conducted in the high TB/HIV-burden countries between 1990 and 2016 on EFV pharmacokinetics during RH coadministration in coinfected patients. Two reviewers conducted article screening and data collection.
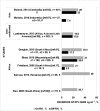
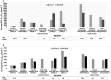
Results: Of 119 records retrieved, 22 were included (two conducted in children), reporting either EFV mid-dose or pre-dose concentrations. In 19 studies, median or mean concentrations of RH range between 1000 and 4000 ng ml-1 , the so-called therapeutic range. The proportion of patients with subtherapeutic concentration of RH ranged between 3.1 and 72.2%, in 12 studies including one conducted in children. The proportion of patients with supratherapeutic concentration ranged from 19.6 to 48.0% in six adult studies and one child study. Five of eight studies reported virological suppression >80%. The association between any grade hepatic and central nervous system adverse effects with EFV/RH interaction was demonstrated in two and three studies, respectively. The frequency of the CYP2B6 516G > T polymorphism ranged from 10 to 28% and was associated with higher plasma EFV concentrations, irrespective of ethnicity.
Conclusions: Anti-TB drug coadministration minimally affect the EFV exposure, efficacy and safety among TB-HIV coinfected African and Asian patients. This supports the current 600 mg EFV dosing when coadministered with anti-TB drugs.
Keywords: HIV/AIDS < infectious diseases; adverse drug reactions; antiretrovirals < infectious diseases; genetics and pharmacogenetics; pharmacokinetics.
© 2018 The British Pharmacological Society.
Figures

References
-
- World Health Organization . Global Health Observatory (GHO) data: HIV/AIDS global situation and trends 2016. Available at http://www.who.int/gho/hiv/en/ (last accessed 13 July 2017).
-
- World Health Organization . Tuberculosis and HIV 2014. Available at http://www.who.int/hiv/topics/tb/about_tb/en/ (last accessed 12 July 2017).
-
- World Health Organization . Global tuberculosis report. Geneva2016. Available at http://www.who.int/tb/publications/global_report/en/ (last accessed 21 June 2017).
-
- World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach Geneva2016. 2nd Available at http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf (last accessed 29 July 2017).
-
- Nelson LJ, Beusenberg M, Habiyambere V, Shaffer N, Vitoria MA, Montero RG, et al Adoption of national recommendations related to use of antiretroviral therapy before and shortly following the launch of the 2013 WHO consolidated guidelines. AIDS 2014; 28 (Suppl 2): S217–S224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

