The N-Methylpyrrolidone (NMP) Effect in Iron-Catalyzed Cross-Coupling with Simple Ferric Salts and MeMgBr
- PMID: 29624838
- PMCID: PMC5992103
- DOI: 10.1002/anie.201802087
The N-Methylpyrrolidone (NMP) Effect in Iron-Catalyzed Cross-Coupling with Simple Ferric Salts and MeMgBr
Abstract
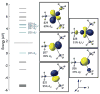
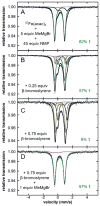
The use of N-methylpyrrolidone (NMP) as a co-solvent in ferric salt catalyzed cross-coupling reactions is crucial for achieving the highly selective, preparative scale formation of cross-coupled product in reactions utilizing alkyl Grignard reagents. Despite the critical importance of NMP, the molecular level effect of NMP on in situ formed and reactive iron species that enables effective catalysis remains undefined. Herein, we report the isolation and characterization of a novel trimethyliron(II) ferrate species, [Mg(NMP)6 ][FeMe3 ]2 (1), which forms as the major iron species in situ in reactions of Fe(acac)3 and MeMgBr under catalytically relevant conditions where NMP is employed as a co-solvent. Importantly, combined GC analysis and 57 Fe Mössbauer spectroscopic studies identified 1 as a highly reactive iron species for the selective formation generating cross-coupled product. These studies demonstrate that NMP does not directly interact with iron as a ligand in catalysis but, alternatively, interacts with the magnesium cations to preferentially stabilize the formation of 1 over [Fe8 Me12 ]- cluster generation, which occurs in the absence of NMP.
Keywords: MCD spectroscopy; Mössbauer spectroscopy; N-methylpyrrolidone; cross-coupling; iron.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sherry BD, Fürstner A. Acc Chem Res. 2008;41:1500–1511. - PubMed
- Fürstner A, Martin R. Chem Lett. 2005;34:624–629.
- Mako TL, Byers JA. Inorg Chem Front. 2016;3:766–790.
- Martin R, Fürstner A. Angew Chem Int Ed. 2004;43:3955–3957. - PubMed
- Carpenter SH, Neidig ML. Isr J Chem. 2017;57:1106–1116. - PMC - PubMed
- Bauer I, Knçlker HJ. Chem Rev. 2015;115:3170–3387. - PubMed
- Fürstner A. ACS Cent Sci. 2016;2:778–789. - PMC - PubMed
-
- Tamura M, Kochi JK. J Am Chem Soc. 1971;93:1487–1489.
- Neumann SM, Kochi JK. J Org Chem. 1975;40:599–606.
-
- Cahiez G, Avedissian H. Synthesis. 1998;1998:1199–1205.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

