Large-Scale Clonal Analysis Resolves Aging of the Mouse Hematopoietic Stem Cell Compartment
- PMID: 29625072
- PMCID: PMC5896201
- DOI: 10.1016/j.stem.2018.03.013
Large-Scale Clonal Analysis Resolves Aging of the Mouse Hematopoietic Stem Cell Compartment
Abstract
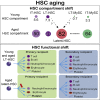
Aging is linked to functional deterioration and hematological diseases. The hematopoietic system is maintained by hematopoietic stem cells (HSCs), and dysfunction within the HSC compartment is thought to be a key mechanism underlying age-related hematopoietic perturbations. Using single-cell transplantation assays with five blood-lineage analysis, we previously identified myeloid-restricted repopulating progenitors (MyRPs) within the phenotypic HSC compartment in young mice. Here, we determined the age-related functional changes to the HSC compartment using over 400 single-cell transplantation assays. Notably, MyRP frequency increased dramatically with age, while multipotent HSCs expanded modestly within the bone marrow. We also identified a subset of functional cells that were myeloid restricted in primary recipients but displayed multipotent (five blood-lineage) output in secondary recipients. We have termed this cell type latent-HSCs, which appear exclusive to the aged HSC compartment. These results question the traditional dogma of HSC aging and our current approaches to assay and define HSCs.
Keywords: HSC; aging; clonal analysis; hematopoiesis; hematopoietic stem cell; multipotency; self-renewal; single cell; stem cell aging.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


References
-
- Carrelha J., Meng Y., Kettyle L.M., Luis T.C., Norfo R., Alcolea V., Boukarabila H., Grasso F., Gambardella A., Grover A. Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature. 2018;554:106–111. - PubMed
-
- Elias H.K., Bryder D., Park C.Y. Molecular mechanisms underlying lineage bias in aging hematopoiesis. Semin. Hematol. 2017;54:4–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

