Gene therapy in rare diseases: the benefits and challenges of developing a patient-centric registry for Strimvelis in ADA-SCID
- PMID: 29625577
- PMCID: PMC5889583
- DOI: 10.1186/s13023-018-0791-9
Gene therapy in rare diseases: the benefits and challenges of developing a patient-centric registry for Strimvelis in ADA-SCID
Abstract
Background: Strimvelis (autologous CD34+ cells transduced to express adenosine deaminase [ADA]) is the first ex vivo stem cell gene therapy approved by the European Medicines Agency (EMA), indicated as a single treatment for patients with ADA-severe combined immunodeficiency (ADA-SCID) who lack a suitable matched related bone marrow donor. Existing primary immunodeficiency registries are tailored to transplantation outcomes and do not capture the breadth of safety and efficacy endpoints required by the EMA for the long-term monitoring of gene therapies. Furthermore, for extended monitoring of Strimvelis, the young age of children treated, small patient numbers, and broad geographic distribution of patients all increase the risk of loss to follow-up before sufficient data have been collected. Establishing individual investigator sites would be impractical and uneconomical owing to the small number of patients from each location receiving Strimvelis.
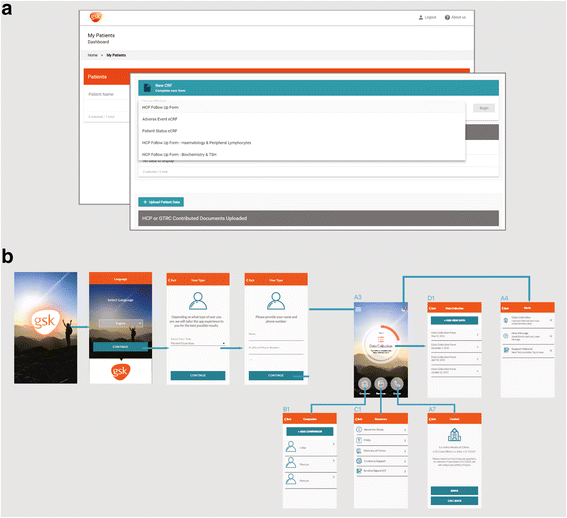
Results: An observational registry has been established to monitor the safety and effectiveness of Strimvelis in up to 50 patients over a minimum of 15 years. To address the potential challenges highlighted above, data will be collected by a single investigator site at Ospedale San Raffaele (OSR), Milan, Italy, and entered into the registry via a central electronic platform. Patients/families and the patient's local physician will also be able to submit healthcare information directly to the registry using a uniquely designed electronic platform. Data entry will be monitored by a Gene Therapy Registry Centre (funded by GlaxoSmithKline) who will ensure that necessary information is collected and flows between OSR, the patient/family and the patient's local healthcare provider.
Conclusion: The Strimvelis registry sets a precedent for the safety monitoring of future gene therapies. A unique, patient-focused design has been implemented to address the challenges of long-term follow-up of patients treated with gene therapy for a rare disease. Strategies to ensure data completeness and patient retention in the registry will help fulfil pharmacovigilance requirements. Collaboration with partners is being sought to expand from a treatment registry into a disease registry. Using practical and cost-efficient approaches, the Strimvelis registry is hoped to encourage further innovation in registry design within orphan drug development.
Keywords: Adenosine deaminase deficiency; Autologous; Gene therapy; Haematopoietic stem cell transplantation; Pharmacovigilance; Severe combined immunodeficiency; Transplantation.
Conflict of interest statement
Authors’ information
SG works in the laboratories overseen by AA at SR-TIGET and is responsible for the performance and technical supervision of cellular assays conducted as part of the follow-up of patients with ADA-SCID treated with gene therapy.
MK was an employee of GlaxoSmithKline, Brentford, Middlesex, UK at the time of the study. Currently an employee of Orchard Therapeutics.
Ethics approval and consent to participate
Ethical approval for the Strimvelis registry has been given by the San Raffaele Hospital Ethics Committee in Italy and will also be obtained where it is required in patients’ own countries. All patients included in the registry are required to provide informed consent to participate.
Consent for publication
Not applicable
Competing interests
MPC is the Principal Investigator (PI) of the ADA-SCID registry trial. AA is the PI of the ADA-SCID gene therapy trial and other clinical trials sponsored by GlaxoSmithKline (GSK) which are part of the alliance between Ospedale San Raffaele-Telethon and GSK. SG, MG, AC and SZ declare no competing interest. MD is an employee of Pharmaceutical Product Development. HS-F, NG, JI, BC, RB, JA and PN are employees of GlaxoSmithKline. MK is an employee of Orchard Therapeutics.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Hershfield M. Adenosine Deaminase Deficiency. NCBI Bookshelf: University of Washington, Seattle; 1993–2017. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

