Interaction between human osteosarcoma and mesenchymal stem cells via an interleukin-8 signaling loop in the tumor microenvironment
- PMID: 29625612
- PMCID: PMC5889532
- DOI: 10.1186/s12964-018-0225-2
Interaction between human osteosarcoma and mesenchymal stem cells via an interleukin-8 signaling loop in the tumor microenvironment
Abstract
Background: Osteosarcoma (OS) is the representative primary malignant bone tumor with the highest incidence. It is known that malignant phenotypes of OS, such as proliferation, invasion, and metastasis, are significantly influenced not only by characteristics of the tumor itself, but also by the surrounding microenvironment. In other words, OS is considered to utilize cells in the vicinity of the tumor by changing the characteristics of these cells. Direct intercellular contact is believed to be important for this phenomenon. In the present study, we hypothesized that an interaction mediated by a humoral factor, requiring no cellular contact, might play a significant role in the progression of OS.
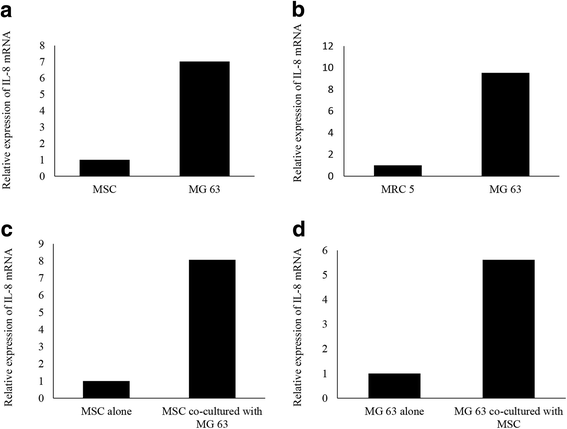
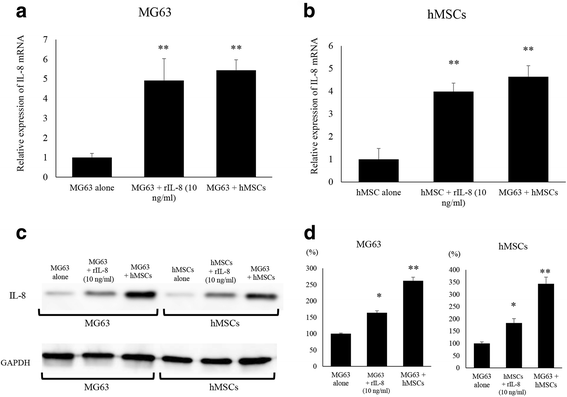
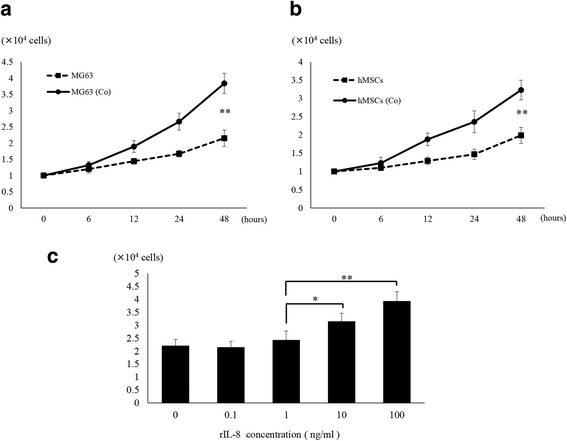
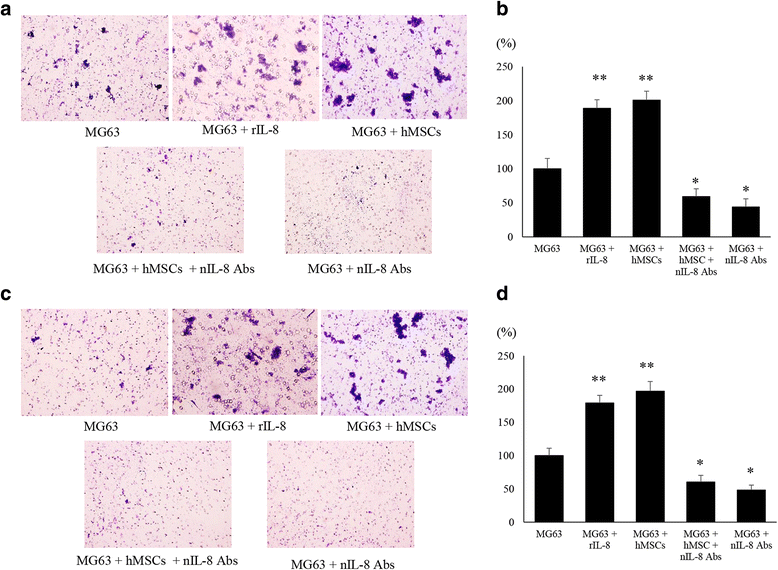
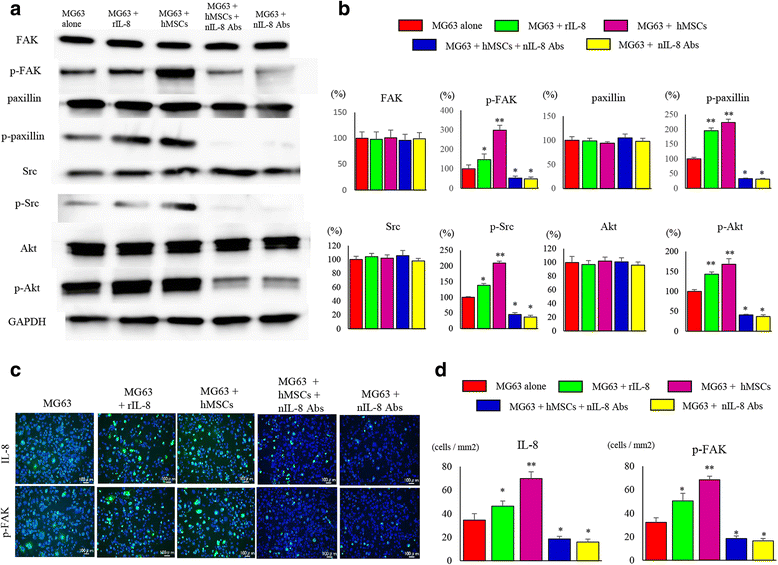
Methods: We developed a new co-culture model, using OS cells and mesenchymal stem cells (MSCs) without cellular contact, and found that both cell types expressed IL-8 at a high level, and FAK in OS cells was phosphorylated leading to an increase in the metastatic potential of the tumor in the co-culture condition.
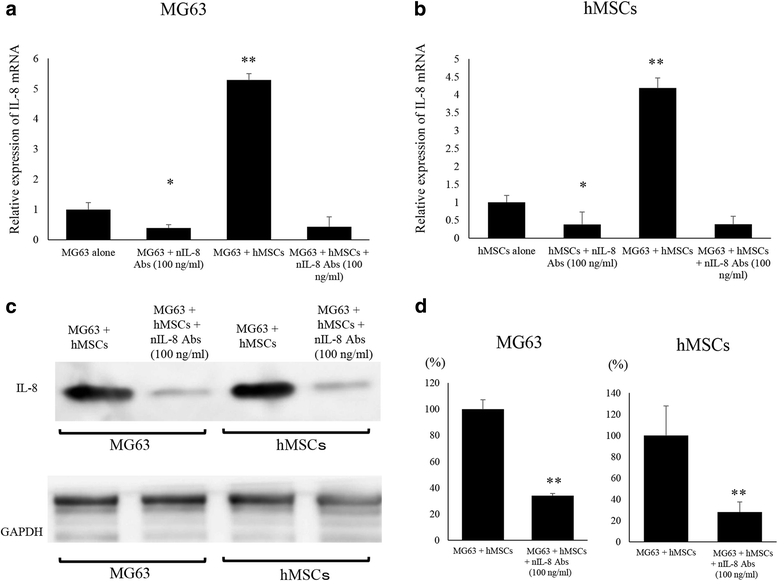
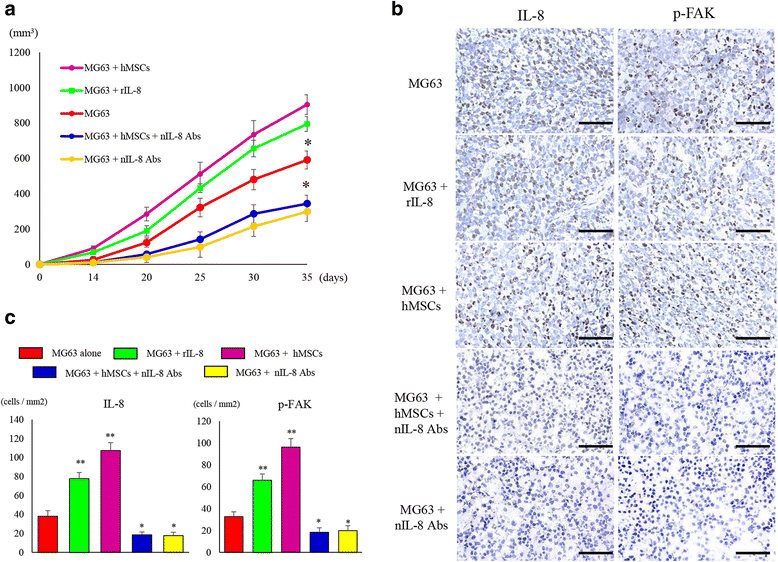
Results: It was revealed that OS cells formed a loop of signal cross-talk in which they released IL-8 as a paracrine factor, stimulating MSCs to express IL-8, and received IL-8 released by MSCs to accelerate IL-8 expression in OS cells. Administration of anti-IL-8 antibody resulted in the inhibition of FAK expression, its downstream signaling, and the invasive potential of the OS cells, resulting in decrease in metastatic lesions.
Conclusion: The present study might lead not only to the clarification of a new molecular mechanism of invasion and metastasis of OS, but also to the development of a new therapeutic strategy of blocking IL-8 in OS.
Keywords: Interleukin-8; Mesenchymal stem cells; Osteosarcoma; Tumor proliferation and metastasis.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was given by the Medical Ethics Committee of Oita University.
Consent for publication
All authors are responsible for the submission of this article and accept the conditions of submission.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
CXCR1/Akt signaling activation induced by mesenchymal stem cell-derived IL-8 promotes osteosarcoma cell anoikis resistance and pulmonary metastasis.Cell Death Dis. 2018 Jun 18;9(7):714. doi: 10.1038/s41419-018-0745-0. Cell Death Dis. 2018. PMID: 29915309 Free PMC article.
-
Human Umbilical Cord Mesenchymal Stem Cells Promote Breast Cancer Metastasis by Interleukin-8- and Interleukin-6-Dependent Induction of CD44(+)/CD24(-) Cells.Cell Transplant. 2015;24(12):2585-99. doi: 10.3727/096368915X687462. Epub 2015 Feb 18. Cell Transplant. 2015. PMID: 25695620
-
STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma.Cancer Lett. 2012 Dec 1;325(1):80-8. doi: 10.1016/j.canlet.2012.06.006. Epub 2012 Jun 26. Cancer Lett. 2012. PMID: 22743617
-
Mesenchymal stem cells in the osteosarcoma microenvironment: their biological properties, influence on tumor growth, and therapeutic implications.Stem Cell Res Ther. 2018 Jan 31;9(1):22. doi: 10.1186/s13287-018-0780-x. Stem Cell Res Ther. 2018. PMID: 29386041 Free PMC article. Review.
-
Mesenchymal Stem Cells and Extracellular Vesicles in Osteosarcoma Pathogenesis and Therapy.Int J Mol Sci. 2021 Oct 13;22(20):11035. doi: 10.3390/ijms222011035. Int J Mol Sci. 2021. PMID: 34681692 Free PMC article. Review.
Cited by
-
Molecular Biology of Osteosarcoma.Cancers (Basel). 2020 Jul 31;12(8):2130. doi: 10.3390/cancers12082130. Cancers (Basel). 2020. PMID: 32751922 Free PMC article. Review.
-
Directing B7-H3 chimeric antigen receptor T cell homing through IL-8 induces potent antitumor activity against pediatric sarcoma.J Immunother Cancer. 2024 Jul 23;12(7):e009221. doi: 10.1136/jitc-2024-009221. J Immunother Cancer. 2024. PMID: 39043604 Free PMC article.
-
Roles of the CXCL8-CXCR1/2 Axis in the Tumor Microenvironment and Immunotherapy.Molecules. 2021 Dec 27;27(1):137. doi: 10.3390/molecules27010137. Molecules. 2021. PMID: 35011369 Free PMC article. Review.
-
Analysis of the signal cross talk via CCL26 in the tumor microenvironment in osteosarcoma.Sci Rep. 2021 Sep 13;11(1):18099. doi: 10.1038/s41598-021-97153-2. Sci Rep. 2021. PMID: 34518591 Free PMC article.
-
Osteoid cell-derived chemokines drive bone-metastatic prostate cancer.Front Oncol. 2023 Mar 21;13:1100585. doi: 10.3389/fonc.2023.1100585. eCollection 2023. Front Oncol. 2023. PMID: 37025604 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

