APTO-253 Is a New Addition to the Repertoire of Drugs that Can Exploit DNA BRCA1/2 Deficiency
- PMID: 29626126
- PMCID: PMC7087411
- DOI: 10.1158/1535-7163.MCT-17-0834
APTO-253 Is a New Addition to the Repertoire of Drugs that Can Exploit DNA BRCA1/2 Deficiency
Abstract
APTO-253 is a small molecule with antiproliferative activity against cell lines derived from a wide range of human malignancies. We sought to determine the mechanisms of action and basis for resistance to APTO-253 so as to identify synthetic lethal interactions that can guide combination studies. The cellular pharmacology of APTO-253 was analyzed in Raji lymphoma cells and a subline selected for resistance (Raji/253R). Using LC/MS/ESI analysis, APTO-253 was found to convert intracellularly to a complex containing one molecule of iron and three molecules of APTO-253 [Fe(253)3]. The intracellular content of Fe(253)3 exceeded that of the native drug by approximately 18-fold, and Fe(253)3 appears to be the most active form. Treatment of cells with APTO-253 caused DNA damage, which led us to ask whether cells deficient in homologous recombination (i.e., loss of BRCA1/2 function) were hypersensitive to this drug. It was found that loss of either BRCA1 or BRCA2 function in multiple isogenic paired cell lines resulted in hypersensitivity to APTO-253 of a magnitude similar to the effects of PARP inhibitors, olaparib. Raji cells selected for 16-fold acquired resistance had 16-fold reduced accumulation of Fe(253)3 RNA-seq analysis revealed that overexpression of the ABCG2 drug efflux pump is a key mechanism of resistance. ABCG2-overexpressed HEK-293 cells were resistant to APTO-253, and inhibition of ABCG2 reversed resistance to APTO-253 in Raji/253R. APTO-253 joins the limited repertoire of drugs that can exploit defects in homologous recombination and is of particular interest because it does not produce myelosuppression. Mol Cancer Ther; 17(6); 1167-76. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
H. Zhang, A. Local, and W.G. Rice are employees of Aptose Biosciences, Inc. S.B. Howell is a consultant of Aptose Biosciences. No potential conflicts of interest were disclosed by the other authors.
Figures

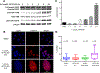
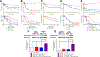
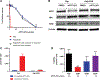
 ), Raji/253R (
), Raji/253R ( ), Raji/253R, and Raji/253R cells after culture in drug-free medium for 3 months (
), Raji/253R, and Raji/253R cells after culture in drug-free medium for 3 months ( ). B, Western blot analysis of proteins involved in apoptosis in Raji and Raji/253R treated with DMSO or APTO-253 0.5 μmol/L for 24 hours. C, The intracellular accumulation of APTO-253 (
). B, Western blot analysis of proteins involved in apoptosis in Raji and Raji/253R treated with DMSO or APTO-253 0.5 μmol/L for 24 hours. C, The intracellular accumulation of APTO-253 ( ) and Fe(253)3 (
) and Fe(253)3 ( ) in Raji and Raji/253R cells after a 6-hour exposure to 0.5 μmol/L APTO-253. D, The intracellular accumulation of Fe(253)3 in the Raji and Raji/253R cells at 6 hours as a function of APTO-253 concentration. Vertical bars, ±SEM. ** P < 0.01; ***, P < 0.001; ****, P < 0.0001.
) in Raji and Raji/253R cells after a 6-hour exposure to 0.5 μmol/L APTO-253. D, The intracellular accumulation of Fe(253)3 in the Raji and Raji/253R cells at 6 hours as a function of APTO-253 concentration. Vertical bars, ±SEM. ** P < 0.01; ***, P < 0.001; ****, P < 0.0001.
 ) and Raji/253R (
) and Raji/253R ( ). D, Concentration–survival curves for Raji (
). D, Concentration–survival curves for Raji ( ) and Raji/253R (
) and Raji/253R ( ) treated with APTO-253 alone or in combination with APTO-253 and 5 nmol/L (
) treated with APTO-253 alone or in combination with APTO-253 and 5 nmol/L ( ) or 50 nmol/L Kol43 (
) or 50 nmol/L Kol43 ( ). E, Cytotoxicity of topotecan in Raji (
). E, Cytotoxicity of topotecan in Raji ( ) and Raji/253R (
) and Raji/253R ( ) and the combination of topotecan and 50 nmol/L Ko143 in Raji/253R (
) and the combination of topotecan and 50 nmol/L Ko143 in Raji/253R ( ). F, Cytotoxicity of carboplatin in Raji (
). F, Cytotoxicity of carboplatin in Raji ( ) and Raji/253R (
) and Raji/253R ( ) and the combination of carboplatin and 50 nmol/L Ko143 in Raji/253R (
) and the combination of carboplatin and 50 nmol/L Ko143 in Raji/253R ( ). G, Concentration–survival curves for HEK-293 transfected with pcDNA (
). G, Concentration–survival curves for HEK-293 transfected with pcDNA ( ) and ABCG2, clone R5 (
) and ABCG2, clone R5 ( ) treated with APTO-253. Vertical bars, ± SEM. **, P < 0.01; **, P < 0.001.
) treated with APTO-253. Vertical bars, ± SEM. **, P < 0.01; **, P < 0.001.References
-
- Mudasir, Wijaya K, Yoshioka N, Inoue H. DNA binding of iron(II) complexes with 1,10-phenanthroline and 4,7-diphenyl-1,10-phenanthroline: salt effect, ligand substituent effect, base pair specificity and binding strength. J Inorg Biochem 2003;94:263–71. - PubMed
-
- Mudasir, Wijaya K, Wahyuni ET, Inoue H, Yoshioka N. Base-specific and enantioselective studies for the DNA binding of iron(II) mixed-ligand complexes containing 1,10-phenanthroline and dipyrido[3,2-a:2′,3′-c]phenazine. Spectrochimica acta Part A, Mol Biomol Spect 2007;66: 163–70. - PubMed
-
- Mudasir, Yoshioka N, Inoue H. Enantioselective DNA binding of iron(II) complexes of methyl-substituted phenanthroline. J Inorg Biochem 2008; 102:1638–43. - PubMed
-
- Mudasir, Yoshioka N, Inoue H. DNA binding of iron(II) mixed-ligand complexes containing 1,10-phenanthroline and 4,7-diphenyl-1,10-phenanthroline. J Inorg Biochem 1999;77:239–47. - PubMed
-
- Huesca M, Lock LS, Khine AA, Viau S, Peralta R, Cukier IH, et al. A novel small molecule with potent anticancer activity inhibits cell growth by modulating intracellular labile zinc homeostasis. Mol Cancer Ther 2009; 8:2586–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

