Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green
- PMID: 29626132
- PMCID: PMC5924901
- DOI: 10.1073/pnas.1718917115
Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green
Abstract
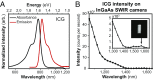
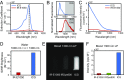
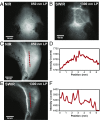
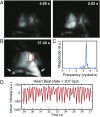
Fluorescence imaging is a method of real-time molecular tracking in vivo that has enabled many clinical technologies. Imaging in the shortwave IR (SWIR; 1,000-2,000 nm) promises higher contrast, sensitivity, and penetration depths compared with conventional visible and near-IR (NIR) fluorescence imaging. However, adoption of SWIR imaging in clinical settings has been limited, partially due to the absence of US Food and Drug Administration (FDA)-approved fluorophores with peak emission in the SWIR. Here, we show that commercially available NIR dyes, including the FDA-approved contrast agent indocyanine green (ICG), exhibit optical properties suitable for in vivo SWIR fluorescence imaging. Even though their emission spectra peak in the NIR, these dyes outperform commercial SWIR fluorophores and can be imaged in the SWIR, even beyond 1,500 nm. We show real-time fluorescence imaging using ICG at clinically relevant doses, including intravital microscopy, noninvasive imaging in blood and lymph vessels, and imaging of hepatobiliary clearance, and show increased contrast compared with NIR fluorescence imaging. Furthermore, we show tumor-targeted SWIR imaging with IRDye 800CW-labeled trastuzumab, an NIR dye being tested in multiple clinical trials. Our findings suggest that high-contrast SWIR fluorescence imaging can be implemented alongside existing imaging modalities by switching the detection of conventional NIR fluorescence systems from silicon-based NIR cameras to emerging indium gallium arsenide-based SWIR cameras. Using ICG in particular opens the possibility of translating SWIR fluorescence imaging to human clinical applications. Indeed, our findings suggest that emerging SWIR-fluorescent in vivo contrast agents should be benchmarked against the SWIR emission of ICG in blood.
Keywords: biomedical imaging; fluorescence imaging; indocyanine green; near infrared; shortwave infrared.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

