High-throughput mouse phenomics for characterizing mammalian gene function
- PMID: 29626206
- PMCID: PMC6582361
- DOI: 10.1038/s41576-018-0005-2
High-throughput mouse phenomics for characterizing mammalian gene function
Abstract
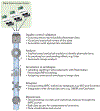
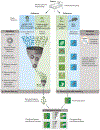
We are entering a new era of mouse phenomics, driven by large-scale and economical generation of mouse mutants coupled with increasingly sophisticated and comprehensive phenotyping. These studies are generating large, multidimensional gene-phenotype data sets, which are shedding new light on the mammalian genome landscape and revealing many hitherto unknown features of mammalian gene function. Moreover, these phenome resources provide a wealth of disease models and can be integrated with human genomics data as a powerful approach for the interpretation of human genetic variation and its relationship to disease. In the future, the development of novel phenotyping platforms allied to improved computational approaches, including machine learning, for the analysis of phenotype data will continue to enhance our ability to develop a comprehensive and powerful model of mammalian gene-phenotype space.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

