LI-RADS 2017: An update
- PMID: 29626376
- PMCID: PMC6652220
- DOI: 10.1002/jmri.26027
LI-RADS 2017: An update
Abstract
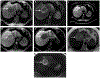
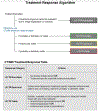
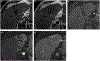
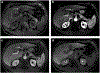
The computed tomography / magnetic resonance imaging (CT/MRI) Liver Imaging Reporting & Data System (LI-RADS) is a standardized system for diagnostic imaging terminology, technique, interpretation, and reporting in patients with or at risk for developing hepatocellular carcinoma (HCC). Using diagnostic algorithms and tables, the system assigns to liver observations category codes reflecting the relative probability of HCC or other malignancies. This review article provides an overview of the 2017 version of CT/MRI LI-RADS with a focus on MRI. The main LI-RADS categories and their application will be described. Changes and updates introduced in this version of LI-RADS will be highlighted, including modifications to the diagnostic algorithm and to the optional application of ancillary features. Comparisons to other major diagnostic systems for HCC will be made, emphasizing key similarities, differences, strengths, and limitations. In addition, this review presents the new Treatment Response algorithm, while introducing the concepts of MRI nonviability and viability. Finally, planned future directions for LI-RADS will be outlined.
Level of evidence: 5 Technical Efficacy: Stage 5 J. Magn. Reson. Imaging 2018;47:1459-1474.
Keywords: LI-RADS; MRI; ancillary features; hepatocellular carcinoma; imaging features; liver.
© 2018 International Society for Magnetic Resonance in Medicine.
Figures




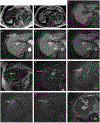
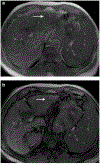




References
-
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. - PubMed
-
- Chernyak V, SantilIan CS, Papadatos D, Sirlin CB. LI-RADS((R)) algorithm: CT and MRI. Abdom Radiol 2018;43:111–126. - PubMed
-
- Tang A, Valasek MA, Sirlin CB. Update on the Liver Imaging Reporting and Data System: What the pathologist needs to know. Adv Anat Pathol. 2015;22:314–322. - PubMed
-
- Sirlin CB. The LI-RADS adventure—a personal statement. Abdom Radiol 2018;43:1–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

