Plasma phospho-tau181 increases with Alzheimer's disease clinical severity and is associated with tau- and amyloid-positron emission tomography
- PMID: 29626426
- PMCID: PMC6097897
- DOI: 10.1016/j.jalz.2018.02.013
Plasma phospho-tau181 increases with Alzheimer's disease clinical severity and is associated with tau- and amyloid-positron emission tomography
Abstract
Introduction: We examined and compared plasma phospho-tau181 (pTau181) and total tau: (1) across the Alzheimer's disease (AD) clinical spectrum; (2) in relation to brain amyloid β (Aβ) positron emission tomography (PET), tau PET, and cortical thickness; and (3) as a screening tool for elevated brain Aβ.
Methods: Participants included 172 cognitively unimpaired, 57 mild cognitively impaired, and 40 AD dementia patients with concurrent Aβ PET (Pittsburgh compound B), tau PET (AV1451), magnetic resonance imaging, plasma total tau, and pTau181.
Results: Plasma total tau and pTau181 levels were higher in AD dementia patients than those in cognitively unimpaired. Plasma pTau181 was more strongly associated with both Aβ and tau PET. Plasma pTau181 was a more sensitive and specific predictor of elevated brain Aβ than total tau and was as good as, or better than, the combination of age and apolipoprotein E (APOE).
Discussion: Plasma pTau181 may have utility as a biomarker of AD pathophysiology and as a noninvasive screener for elevated brain Aβ.
Keywords: Alzheimer's disease; Amyloid PET; Plasma phosphorylated tau; Plasma tau; Predicting brain amyloid; Tau PET.
Copyright © 2018 the Alzheimer's Association. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
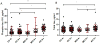
References
-
- Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–63. - PubMed
-
- Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG041851/AG/NIA NIH HHS/United States
- R01 AG029550/AG/NIA NIH HHS/United States
- R01 AG056366/AG/NIA NIH HHS/United States
- R01 AG034676/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- R01 AG011378/AG/NIA NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- R01 AG037551/AG/NIA NIH HHS/United States
- R01 AG032306/AG/NIA NIH HHS/United States
- R01 AG043392/AG/NIA NIH HHS/United States
- R01 NS097495/NS/NINDS NIH HHS/United States
- U01 AG006786/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- U01 HL096917/HL/NHLBI NIH HHS/United States
- P50 AG044170/AG/NIA NIH HHS/United States
- R01 AG049704/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

