Hypoxia-selective allosteric destabilization of activin receptor-like kinases: A potential therapeutic avenue for prophylaxis of heterotopic ossification
- PMID: 29626545
- PMCID: PMC9851731
- DOI: 10.1016/j.bone.2018.03.027
Hypoxia-selective allosteric destabilization of activin receptor-like kinases: A potential therapeutic avenue for prophylaxis of heterotopic ossification
Abstract
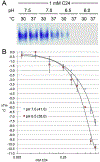
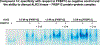
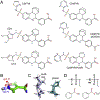
Heterotopic ossification (HO), the pathological extraskeletal formation of bone, can arise from blast injuries, severe burns, orthopedic procedures and gain-of-function mutations in a component of the bone morphogenetic protein (BMP) signaling pathway, the ACVR1/ALK2 receptor serine-threonine (protein) kinase, causative of Fibrodysplasia Ossificans Progressiva (FOP). All three ALKs (-2, -3, -6) that play roles in bone morphogenesis contribute to trauma-induced HO, hence are well-validated pharmacological targets. That said, development of inhibitors, typically competitors of ATP binding, is inherently difficult due to the conserved nature of the active site of the 500+ human protein kinases. Since these enzymes are regulated via inherent plasticity, pharmacological chaperone-like drugs binding to another (allosteric) site could hypothetically modulate kinase conformation and activity. To test for such a mechanism, a surface pocket of ALK2 kinase formed largely by a key allosteric substructure was targeted by supercomputer docking of drug-like compounds from a virtual library. Subsequently, the effects of docked hits were further screened in vitro with purified recombinant kinase protein. A family of compounds with terminal hydrogen-bonding acceptor groups was identified that significantly destabilized the protein, inhibiting activity. Destabilization was pH-dependent, putatively mediated by ionization of a histidine within the allosteric substructure with decreasing pH. In vivo, nonnative proteins are degraded by proteolysis in the proteasome complex, or cellular trashcan, allowing for the emergence of therapeutics that inhibit through degradation of over-active proteins implicated in the pathology of diseases and disorders. Because HO is triggered by soft-tissue trauma and ensuing hypoxia, dependency of ALK destabilization on hypoxic pH imparts selective efficacy on the allosteric inhibitors, providing potential for safe prophylactic use.
Keywords: ACVR1; ALK2; Allosteric; BMP; BMPRII; Bone morphogenetic protein; FKBP12; FOP; Fibrodysplasia ossificans progressiva; Heterotopic ossification; Hydrophobic tagging; Hypoxia; Hypoxic pH; PROTACs; Pharmacological chaperone; Protein kinase; R-spine; Selective degrader; Small molecule kinase inhibitor; αC-β4 loop.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures

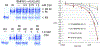



Similar articles
-
Alk2 regulates early chondrogenic fate in fibrodysplasia ossificans progressiva heterotopic endochondral ossification.Stem Cells. 2014 May;32(5):1289-300. doi: 10.1002/stem.1633. Stem Cells. 2014. PMID: 24449086 Free PMC article.
-
An ALK2 inhibitor, BLU-782, prevents heterotopic ossification in a mouse model of fibrodysplasia ossificans progressiva.Sci Transl Med. 2024 May 29;16(749):eabp8334. doi: 10.1126/scitranslmed.abp8334. Epub 2024 May 29. Sci Transl Med. 2024. PMID: 38809966
-
Effects of FKBP12 and type II BMP receptors on signal transduction by ALK2 activating mutations associated with genetic disorders.Bone. 2018 Jun;111:101-108. doi: 10.1016/j.bone.2018.03.015. Epub 2018 Mar 15. Bone. 2018. PMID: 29551750
-
The role of Activin A in fibrodysplasia ossificans progressiva: a prominent mediator.Biosci Rep. 2019 Aug 2;39(8):BSR20190377. doi: 10.1042/BSR20190377. Print 2019 Aug 30. Biosci Rep. 2019. PMID: 31341010 Free PMC article. Review.
-
Development of New Therapeutic Agents for Fibrodysplasia Ossificans Progressiva.Curr Mol Med. 2016;16(1):4-11. doi: 10.2174/1566524016666151222142446. Curr Mol Med. 2016. PMID: 26695699 Review.
Cited by
-
Polypeptide Substrate Accessibility Hypothesis: Gain-of-Function R206H Mutation Allosterically Affects Activin Receptor-like Protein Kinase Activity.Biomolecules. 2023 Jul 14;13(7):1129. doi: 10.3390/biom13071129. Biomolecules. 2023. PMID: 37509165 Free PMC article.
-
The hypoxic microenvironment: a driving force for heterotopic ossification progression.Cell Commun Signal. 2020 Feb 7;18(1):20. doi: 10.1186/s12964-020-0509-1. Cell Commun Signal. 2020. PMID: 32028956 Free PMC article. Review.
-
The PROTAC technology in drug development.Cell Biochem Funct. 2019 Jan;37(1):21-30. doi: 10.1002/cbf.3369. Epub 2019 Jan 2. Cell Biochem Funct. 2019. PMID: 30604499 Free PMC article. Review.
-
Macrophages in heterotopic ossification: from mechanisms to therapy.NPJ Regen Med. 2021 Oct 26;6(1):70. doi: 10.1038/s41536-021-00178-4. NPJ Regen Med. 2021. PMID: 34702860 Free PMC article. Review.
-
Muscle injury-induced hypoxia alters the proliferation and differentiation potentials of muscle resident stromal cells.Skelet Muscle. 2019 Jun 19;9(1):18. doi: 10.1186/s13395-019-0202-5. Skelet Muscle. 2019. PMID: 31217019 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

