Plasma mSEPT9: A Novel Circulating Cell-free DNA-Based Epigenetic Biomarker to Diagnose Hepatocellular Carcinoma
- PMID: 29627389
- PMCID: PMC5952996
- DOI: 10.1016/j.ebiom.2018.03.029
Plasma mSEPT9: A Novel Circulating Cell-free DNA-Based Epigenetic Biomarker to Diagnose Hepatocellular Carcinoma
Abstract
Background: Patients with cirrhosis are at high risk of hepatocellular carcinoma (HCC). The SEPT9 gene is a key regulator of cell division and tumor suppressor whose hypermethylation is associated with liver carcinogenesis. The primary aim of this study was to evaluate the diagnostic accuracy of a PCR-based assay for the analysis of SEPT9 promoter methylation in circulating cell-free DNA (mSEPT9) for diagnosing HCC among cirrhotic patients.
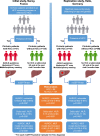
Methods: We report two phase II biomarker studies that included cirrhotic patients with or without HCC from France (initial study) and Germany (replication study). All patients received clinical and biological evaluations, and liver imaging according to current recommendations. The primary outcome was defined as the presence of HCC according to guidelines from the American Association for the Study of Liver Diseases. The diagnosis of HCC was confirmed by abdominal contrast-enhanced computed tomography scan and systematically discussed in a multidisciplinary consultation meeting. HCC-free cirrhotic patients were recruited if the screening abdominal ultrasound showed no evidence of HCC at the time of blood sampling for the mSEPT9 test and on the next visit six months later. The adjudicating physicians were blinded to patient results associated with the mSEPT9 test.
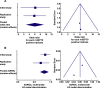
Findings: We included 289 patients with cirrhosis (initial: 186; replication: 103), among whom 98 had HCC (initial: 51; replication: 47). The mSEPT9 test exhibited high diagnostic accuracy for HCC diagnosis, with an area under the receiver operating characteristic curve (AUROC) of 0.944 (0.900-0.970, p<0.0001) in the initial study (replication: 0.930 [0.862-0.971, p<0.0001]; meta-analysis: AUROC=0.940 [0.910-0.970, p<0.0001], no heterogeneity: I2=0%, p=0.67; and no publication bias). In multivariate logistic regression analysis, the number of positive mSEPT9 triplicates was the only independent variable significantly associated with HCC diagnosis (initial: OR=6.30, for each mSEPT9 positive triplicate [2.92-13.61, p<0.0001]; replication: OR=6.07 [3.25-11.35, p<0.0001]; meta-analysis: OR=6.15 [2.93-9.38, p<0.0001], no heterogeneity: I2=0%, p=0.95; no publication bias). AUROC associated with the discrimination of the logistic regression models in initial and validation studies were 0.969 (0.930-0.989) and 0.942 (0.878-0.978), respectively, with a pooled AUROC of 0.962 ([0.937-0.987, p<0.0001], no heterogeneity: I2=0%, p=0.36; and no publication bias).
Interpretation: Among patients with cirrhosis, the mSEPT9 test constitutes a promising circulating epigenetic biomarker for HCC diagnosis at the individual patient level. Future prospective studies should assess the mSEPT9 test in the screening algorithm for cirrhotic patients to improve risk prediction and personalized therapeutic management of HCC.
Keywords: Circulating cell-free DNA-based epigenetic biomarker; Cirrhosis; DNA methylation; Hepatocellular carcinoma; mSEPT9.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Akiyama J., Alexandre L., Baruah A. Strategy for prevention of cancers of the esophagus. Ann. N. Y. Acad. Sci. 2014;1325:108–126. - PubMed
-
- Berhane S., Toyoda H., Tada T. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin. Gastroenterol. Hepatol. 2016;14:875–886. (e6) - PubMed
-
- Borenstein M., Hedges L.V., Higgins J., Rothstein H.R. Introduction to Meta-Analysis. 2009. Generality of the basic inverse-variance method; pp. 311–319.
-
- Bruix J., Reig M., Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

