A Jagged 1-Notch 4 molecular switch mediates airway inflammation induced by ultrafine particles
- PMID: 29627423
- PMCID: PMC6173656
- DOI: 10.1016/j.jaci.2018.03.009
A Jagged 1-Notch 4 molecular switch mediates airway inflammation induced by ultrafine particles
Abstract
Background: Exposure to traffic-related particulate matter promotes asthma and allergic diseases. However, the precise cellular and molecular mechanisms by which particulate matter exposure acts to mediate these effects remain unclear.
Objective: We sought to elucidate the cellular targets and signaling pathways critical for augmentation of allergic airway inflammation induced by ambient ultrafine particles (UFP).
Methods: We used in vitro cell-culture assays with lung-derived antigen-presenting cells and allergen-specific T cells and in vivo mouse models of allergic airway inflammation with myeloid lineage-specific gene deletions, cellular reconstitution approaches, and antibody inhibition studies.
Results: We identified lung alveolar macrophages (AM) as the key cellular target of UFP in promoting airway inflammation. Aryl hydrocarbon receptor-dependent induction of Jagged 1 (Jag1) expression in AM was necessary and sufficient for augmentation of allergic airway inflammation by UFP. UFP promoted TH2 and TH17 cell differentiation of allergen-specific T cells in a Jag1- and Notch 4-dependent manner. Treatment of mice with an anti-Notch 4 antibody abrogated exacerbation of allergic airway inflammation induced by UFP.
Conclusion: UFP exacerbate allergic airway inflammation by promoting a Jag1-Notch 4-dependent interaction between AM and allergen-specific T cells, leading to augmented TH cell differentiation.
Keywords: Airway hyperresponsiveness; Jagged 1; Notch; Notch 4; allergic airway inflammation; alveolar macrophages; aryl hydrocarbon receptor; asthma; traffic-related particulate matter; ultrafine particles.
Copyright © 2018 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures



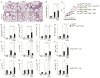
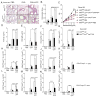


References
-
- Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70:245–56. - PubMed
-
- Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Bruske I, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3:933–42. - PubMed
-
- Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ Int. 2017;100:1–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

