Poloxamer 188 decreases membrane toxicity of mutant SOD1 and ameliorates pathology observed in SOD1 mouse model for ALS
- PMID: 29627580
- PMCID: PMC5943144
- DOI: 10.1016/j.nbd.2018.03.014
Poloxamer 188 decreases membrane toxicity of mutant SOD1 and ameliorates pathology observed in SOD1 mouse model for ALS
Abstract
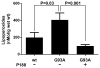
Here we report a gain in function for mutant (mt) superoxide dismutase I (SOD1), a cause of familial amyotrophic lateral sclerosis (FALS), wherein small soluble oligomers of mtSOD1 acquire a membrane toxicity. Phosphatidylglycerol (PG) lipid domains are selectively targeted, which could result in membrane damage or "toxic channels" becoming active in the bilayer. This PG-selective SOD1-mediated membrane toxicity is largely reversible in vitro by a widely-available FDA-approved surfactant and membrane-stabilizer P188. Treatment of G93ASOD1 transgenic mice with P188 significantly delayed symptoms onset, extended survival and decreased motoneuron death. The use of P188 or an analogue, which targets mtSOD1 misfolding-induced membrane toxicity, may provide a new direction for ALS treatment.
Keywords: AFM; ALS; Atomic force microscopy; Electrophysiology; F68; G93A; Lipid peroxidation; Membrane toxicity; P188; Poloxamer; Protein misfolding; SOD1; Superoxide dismutase.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

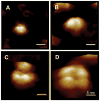

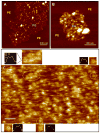

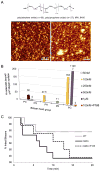


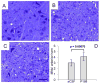
References
-
- Banquy X, Lee DW, Kristiansen K, Gebbie MA, Israelachvili JN. Interaction forces between supported lipid bilayers in the presence of PEGylated polymers. Biomacromolecules. 2015;17(1):88–97. - PubMed
-
- Barber SC, Mead RJ, Shaw PJ. Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2006;1762(11):1051–1067. - PubMed
-
- Beal MF. Mitochondria and the pathogenesis of ALS. Brain. 2000;123(7):1291–1292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

