Sulfidic Anion Concentrations on Early Earth for Surficial Origins-of-Life Chemistry
- PMID: 29627997
- PMCID: PMC6225604
- DOI: 10.1089/ast.2017.1770
Sulfidic Anion Concentrations on Early Earth for Surficial Origins-of-Life Chemistry
Abstract
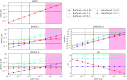
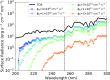
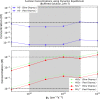
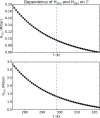
A key challenge in origin-of-life studies is understanding the environmental conditions on early Earth under which abiogenesis occurred. While some constraints do exist (e.g., zircon evidence for surface liquid water), relatively few constraints exist on the abundances of trace chemical species, which are relevant to assessing the plausibility and guiding the development of postulated prebiotic chemical pathways which depend on these species. In this work, we combine literature photochemistry models with simple equilibrium chemistry calculations to place constraints on the plausible range of concentrations of sulfidic anions (HS-, HSO3-, SO32-) available in surficial aquatic reservoirs on early Earth due to outgassing of SO2 and H2S and their dissolution into small shallow surface water reservoirs like lakes. We find that this mechanism could have supplied prebiotically relevant levels of SO2-derived anions, but not H2S-derived anions. Radiative transfer modeling suggests UV light would have remained abundant on the planet surface for all but the largest volcanic explosions. We apply our results to the case study of the proposed prebiotic reaction network of Patel et al. ( 2015 ) and discuss the implications for improving its prebiotic plausibility. In general, epochs of moderately high volcanism could have been especially conducive to cyanosulfidic prebiotic chemistry. Our work can be similarly applied to assess and improve the prebiotic plausibility of other postulated surficial prebiotic chemistries that are sensitive to sulfidic anions, and our methods adapted to study other atmospherically derived trace species.
Keywords: Early Earth; Origin of life; Planetary environments; Prebiotic chemistry; UV radiation; Volcanism.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Akoopie A. and Müller U.F. (2016) Lower temperature optimum of a smaller, fragmented triphosphorylation ribozyme. Phys Chem Chem Phys 18:20118–20125 - PubMed
-
- Amend J.P. and McCollom T.M. (2009) Energetics of biomolecule synthesis on early Earth. In Chemical Evolution II: From the Origins of Life to Modern Society, edited by Zaikowski L., Friedrich J.M., and Seidel S.R., American Chemical Society, Washington, DC, pp 63–94
-
- Amshoff P., Weger T., and Ostertag-Henning C. (2016) SO2 solution, hydrolysis and disproportionation at geological storage conditions in the system CO2-SO2-H2O. In Goldschmidt Conference Abstracts
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
