Distribution of rotavirus genotypes associated with acute diarrhoea in Zimbabwean children less than five years old before and after rotavirus vaccine introduction
- PMID: 29628149
- PMCID: PMC11974498
- DOI: 10.1016/j.vaccine.2018.03.069
Distribution of rotavirus genotypes associated with acute diarrhoea in Zimbabwean children less than five years old before and after rotavirus vaccine introduction
Abstract
Background: Sentinel surveillance for diarrhoea is important to monitor changes in rotavirus epidemiological trends and circulating genotypes among children under 5 years before and after vaccine introduction. The Zimbabwe Ministry of Health and Child Care introduced rotavirus vaccine in national immunization program in May 2014.
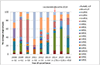
Methods: Active hospital-based surveillance for diarrhoea was conducted at 3 sentinel sites from 2008 to 2016. Children aged less than 5 years, who presented with acute gastroenteritis as a primary illness and who were admitted to a hospital ward or treated at the emergency unit, were enrolled and had a stool specimen collected and tested for rotavirus by enzyme immunoassay (EIA). Genotyping of positive stools was performed using reverse-transcription polymerase chain reaction and genotyping assays. Pre-vaccine introduction, 10% of all positive stool specimens were genotyped and all adequate positive stools were genotyped post-vaccine introduction.
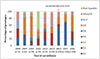
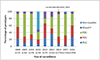
Results: During the pre-vaccine period, a total of 6491 acute gastroenteritis stools were collected, of which 3016 (46%) tested positive for rotavirus and 312 (10%) of the rotavirus positive stools were genotyped. During the post-vaccine period, a total of 3750 acute gastroenteritis stools were collected, of which 937 (25%) tested positive for rotavirus and 784 (84%) were genotyped. During the pre-vaccine introduction the most frequent genotype was G9P[8] (21%) followed by G2P[4] (12%), G1P[8] (6%), G2P[6] (5%), G12P[6] (4%), G9P[6] (3%) and G8P[4] (3%). G1P[8] (30%) was most dominant two years after vaccine introduction followed by G9P[6] (20%), G2P[4] (15%), G9P[8] (11%) and G1P[6] (4%).
Conclusion: The decline in positivity rate is an indication of early vaccine impact. Diversity of circulating strains underscores the importance of continued monitoring and strain surveillance after vaccine introduction.
Keywords: Genotypes vaccine; Rotavirus; Surveillance; Zimbabwe.
Copyright © 2018. Published by Elsevier Ltd.
Figures

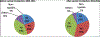

References
-
- World Health Organization. Introduction of rotavirus vaccines into national immunization programmes - Management manual, WHO Document Production Services, Geneva, Switzerland, 2009.
-
- Ahmed SF, Mansour AM, Klena JD, Husain TS, Hassan KA, Mohamed F and Steele AD. Rotavirus genotypes associated with acute diarrhoea. Pediatr Infect Dis J 2014;33:S62–S68. - PubMed
-
- Bourdett-Stanziola L, Jiménez C, and Ortega-Barria E. Diversity of Human Rotavirus G and P Genotypes in Panama, Costa Rica, and the Dominican Republic. The Am J Tropic Med Hygien 2008; 79:921–924. - PubMed
-
- World Health Organization,Estimated rotavirus death for children under 5 years of age: http://www.who.int/immunization/monitoring_surveillance/burden/estimates... [accessed 25.05.2017].
-
- Seheri M, Nemarude L, Peenze I, Netshifhefhe L, Nyaga MM, Ngobeni HG, Maphalala G, Maake LL, Steele AD, Mwenda JM and Mphahlele JM. Update of rotavirus strain circulating in Africa from 2007 through 2011. Pediatr Infect Dis J 2014; 33:S76–S84. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

