ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial
- PMID: 29628312
- PMCID: PMC6089612
- DOI: 10.1016/S1470-2045(18)30148-7
ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial
Abstract
Background: Immunotherapy with PD-1 or PD-L1 blockade fails to induce a response in about 80% of patients with unselected non-small cell lung cancer (NSCLC), and many of those who do initially respond then develop resistance to treatment. Agonists that target the shared interleukin-2 (IL-2) and IL-15Rβγ pathway have induced complete and durable responses in some cancers, but no studies have been done to assess the safety or efficacy of these agonists in combination with anti-PD-1 immunotherapy. We aimed to define the safety, tolerability, and activity of this drug combination in patients with NSCLC.
Methods: In this non-randomised, open-label, phase 1b trial, we enrolled patients (aged ≥18 years) with previously treated histologically or cytologically confirmed stage IIIB or IV NSCLC from three academic hospitals in the USA. Key eligibility criteria included measurable disease, eligibility to receive anti-PD-1 immunotherapy, and an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients received the anti-PD-1 monoclonal antibody nivolumab intravenously at 3 mg/kg (then 240 mg when US Food and Drug Administration [FDA]-approved dosing changed) every 14 days (either as new treatment or continued treatment at the time of disease progression) and the IL-15 superagonist ALT-803 subcutaneously once per week on weeks 1-5 of four 6-week cycles for 6 months. ALT-803 was administered at one of four escalating dose concentrations: 6, 10, 15, or 20 μg/kg. The primary endpoint was to define safety and tolerability and to establish a recommended phase 2 dose of ALT-803 in combination with nivolumab. Analyses were per-protocol and included any patients who received at least one dose of study treatment. This trial is registered with ClinicalTrials.gov, number NCT02523469; phase 2 enrolment of patients is ongoing.
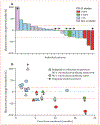
Findings: Between Jan 18, 2016, and June 28, 2017, 23 patients were enrolled and 21 were treated at four dose levels of ALT-803 in combination with nivolumab. Two patients did not receive treatment because of the development of inter-current illness during enrolment, one patient due to leucopenia and one patient due to pulmonary dysfunction. No dose-limiting toxicities were recorded and the maximum tolerated dose was not reached. The most common adverse events were injection-site reactions (in 19 [90%] of 21 patients) and flu-like symptoms (15 [71%]). The most common grade 3 adverse events, occurring in two patients each, were lymphocytopenia and fatigue. A grade 3 myocardial infarction occurred in one patient. No grade 4 or 5 adverse events were recorded. The recommended phase 2 dose of ALT-803 is 20 μg/kg given once per week subcutaneously in combination with 240 mg intravenous nivolumab every 2 weeks.
Interpretation: ALT-803 in combination with nivolumab can be safely administered in an outpatient setting. The promising clinical activity observed with the addition of ALT-803 to the regimen of patients with PD-1 monoclonal antibody relapsed and refractory disease shows evidence of anti-tumour activity for a new class of agents in NSCLC.
Funding: Altor BioScience (a NantWorks company), National Institutes of Health, and Medical University of South Carolina Hollings Cancer Center.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
JMW reports grants from Altor Bioscience, during the conduct of the study. VV reports personal fees from Genentech, Merck, Bristol-Myers Squibb, Takeda, and Celgene, and grants and personal fees from AstraZeneca, outside the submitted work. JGR reports other from Imbio LLC, outside the submitted work. JSM reports grants from Altor Biosciences, outside the submitted work. JAR reports personal fees from Adaptive Biotechnologies, outside the submitted work. CMS reports personal fees from Adaptive Biotechnologies, outside the submitted work. ECY reports personal fees from Adaptive Biotechnologies, outside the submitted work. WLR reports grants from Bristol-Myers Squibb, Tesaro, MedImmune, Nektar Therapeutics, Aeglea Biotherapeutics, IRX Therapeutics, Merck, and Galectin Therapeutics, outside the submitted work. JOE is an employee and equity holder of Altor Bioscience. PRR is an employee and equity holder of Altor Bioscience and has a patent ALT-803 issued, and a patent ALT-803 plus checkpoint inhibitors pending. EKJ is an employee and equity holder of Altor Bioscience. ADR reports personal fees from Altor Bioscience, during the conduct of the study; and personal fees from Altor BioScience, outside the submitted work. HCW is an employee and equity holder of Altor Bioscience and has a patent ALT-803 issued, and a patent ALT-803 plus checkpoint inhibitors pending. MPR reports grants from Altor Bioscience, XOMA, and Neumedicines, outside the submitted work. In addition, MPR has a patent related to IL-2/anti-IL-2 monoclonal antibody complexes pending. All other authors declare no competing interests.
Figures


Comment in
-
Combining top-ranked immunotherapeutics in lung cancer.Lancet Oncol. 2018 May;19(5):592-594. doi: 10.1016/S1470-2045(18)30186-4. Epub 2018 Apr 5. Lancet Oncol. 2018. PMID: 29628313 No abstract available.
References
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al., and the KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–33. - PubMed
-
- Langer CJ, Gadgeel SM, Borghaei H, et al., and the KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17: 1497–508. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

