Structural analyses of Arabidopsis thaliana legumain γ reveal differential recognition and processing of proteolysis and ligation substrates
- PMID: 29628443
- PMCID: PMC5995516
- DOI: 10.1074/jbc.M117.817031
Structural analyses of Arabidopsis thaliana legumain γ reveal differential recognition and processing of proteolysis and ligation substrates
Abstract
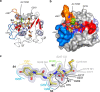
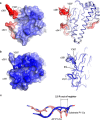
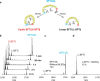
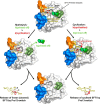
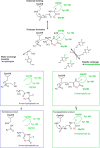
Legumain is a dual-function protease-peptide ligase whose activities are of great interest to researchers studying plant physiology and to biotechnological applications. However, the molecular mechanisms determining the specificities for proteolysis and ligation are unclear because structural information on the substrate recognition by a fully activated plant legumain is unavailable. Here, we present the X-ray structure of Arabidopsis thaliana legumain isoform γ (AtLEGγ) in complex with the covalent peptidic Ac-YVAD chloromethyl ketone (CMK) inhibitor targeting the catalytic cysteine. Mapping of the specificity pockets preceding the substrate-cleavage site explained the known substrate preference. The comparison of inhibited and free AtLEGγ structures disclosed a substrate-induced disorder-order transition with synergistic rearrangements in the substrate-recognition sites. Docking and in vitro studies with an AtLEGγ ligase substrate, sunflower trypsin inhibitor (SFTI), revealed a canonical, protease substrate-like binding to the active site-binding pockets preceding and following the cleavage site. We found the interaction of the second residue after the scissile bond, P2'-S2', to be critical for deciding on proteolysis versus cyclization. cis-trans-Isomerization of the cyclic peptide product triggered its release from the AtLEGγ active site and prevented inadvertent cleavage. The presented integrative mechanisms of proteolysis and ligation (transpeptidation) explain the interdependence of legumain and its preferred substrates and provide a rational framework for engineering optimized proteases, ligases, and substrates.
Keywords: chemical biology; computational biology; crystal structure; cysteine protease; pH regulation; peptide biosynthesis; plant biochemistry; structural biology; transpeptidation; water displacement model.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Gillon A. D., Saska I., Jennings C. V., Guarino R. F., Craik D. J., and Anderson M. A. (2008) Biosynthesis of circular proteins in plants. Plant J. 53, 505–515 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

