Polarisation effects on the solvation properties of alcohols
- PMID: 29629077
- PMCID: PMC5875020
- DOI: 10.1039/c7sc04890d
Polarisation effects on the solvation properties of alcohols
Abstract
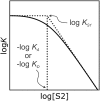
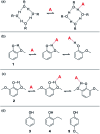
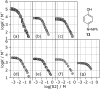
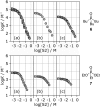
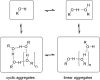
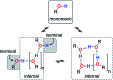
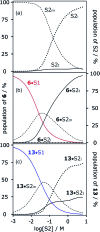
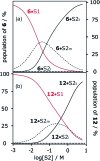
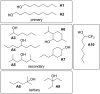
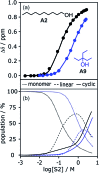
Alcohol solvents are significantly more polar than expected based on the measured H-bonding properties of monomeric alcohols in dilute solution. Self-association of alcohols leads to formation of cyclic aggregates and linear polymeric chains that have a different polarity from the alcohol monomer. Cyclic aggregates are less polar than the monomer, and the chain ends of linear polymers are more polar. The solvation properties of alcohols therefore depend on the interplay of these self-association equilibria and the equilibria involving interactions with solutes. Twenty-one different molecular recognition probes of varying polarity were used to probe the solvation properties of alkane-alcohol mixtures across a wide range of different solvent compositions. The results allow dissection of the complex equilibria present in these systems. Formation of a H-bond between two alcohol molecules leads to polarisation of the hydroxyl groups, resulting in an increase in binding affinity for subsequent interactions with the unbound donor and acceptor sites. The H-bond donor parameter (α) for these sites increases from 2.7 to 3.5, and the H-bond acceptor parameter (β) increases from 5.3 to 6.9. Polarisation is a short range effect limited to the first H-bond in a chain, and formation of subsequent H-bonds in longer chains does not further increase the polarity of chain ends. H-bond donor sites involved in a H-bond are unavailable for further interactions, because the formation of a bifurcated three-centre H-bond is three orders of magnitude less favourable than formation of a conventional two-centre H-bond. These findings are reproduced by quantum chemical calculations of the molecular electrostatic potential surfaces of alcohol aggregates. Thus, the overall solvation properties of alcohols depend on the speciation of different aggregates, the polarities of these species and the polarities of the solutes. At low alcohol concentrations, polar solutes are solvated by alcohol monomers, and at higher alcohol concentrations, solutes are solvated by the more polar chain ends of linear polymers. The less polar cyclic aggregates are less important for interactions with solutes. Similar behavior was found for ten different alcohol solvents. Tertiary alcohols are marginally less polar solvents than primary alcohols, due to steric interactions that destabilises the formation of polymeric aggregates leading to lower concentrations of polar chain ends. One alcohol with an electron-withdrawing substituent was studied, and this solvent showed slightly different behavior, because the H-bond donor and acceptor properties are different.
Figures






References
-
- Abraham M. H., Grellier P. L., Prior D. V., Duce P. P., Morris J. J., Taylor P. J. J. Chem. Soc., Perkin Trans. 2. 1989;(6):699–711.
-
- Abraham M. H., Grellier P. L., Prior D. V., Morris J. J., Taylor P. J. J. Chem. Soc., Perkin Trans. 2. 1990;4:521–529.
-
- Hunter C. A. Angew. Chem., Int. Ed. 2004;43(40):5310–5324. - PubMed
-
- Abraham M. H., Buist G. J., Grellier P. L., McGill R. A., Prior D., Oliver S., Turner E., Morris J. J., Taylor P. J., Nicolet P., Maria P. C., Gal J. F., Abboud J. L. M., Doherty R. M., Kamlet M. J., Shuely W. J., Taft R. W. J. Phys. Org. Chem. 1989;2(7):540–552.
-
- Cabot R., Hunter C. A. Chem. Soc. Rev. 2012;41(9):3485–3492. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

