Site-selective bromination of sp3 C-H bonds
- PMID: 29629078
- PMCID: PMC5873043
- DOI: 10.1039/c7sc04611a
Site-selective bromination of sp3 C-H bonds
Abstract
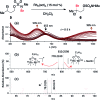
A method for converting sp3 C-H to C-Br bonds using an N-methyl sulfamate directing group is described. The reaction employs Rh2(oct)4 and a mixture of NaBr and NaOCl and is performed in aqueous solution open to air. For all sulfamates examined, oxidation occurs with high selectivity at the γ-carbon, affording a uniquely predictable method for C-H bond halogenation. Results from a series of mechanistic experiments suggest that substrate oxidation likely proceeds by a radical chain process. Initial formation of an N-halogenated sulfamate followed by Rh-mediated homolysis generates an N-centered radical, which serves as the active oxidant.
Figures


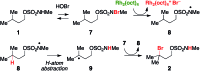



References
-
- Mack J. B. C., Gipson J. D., Du Bois J., Sigman M. S. J. Am. Chem. Soc. 2017;139:9503–9506. - PubMed
-
- White M. C. Science. 2012;335:807–809. - PubMed
-
- Company A., Lloret J., Gómez L. and Costas M., in Alkane C–H Activation by Single-Site Metal Catalysis, ed. P. J. Pérez, Springer Netherlands, Dordrecht, 2012, pp. 143–228, 10.1007/978-90-481-3698-8_5. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

