MoleculeNet: a benchmark for molecular machine learning
- PMID: 29629118
- PMCID: PMC5868307
- DOI: 10.1039/c7sc02664a
MoleculeNet: a benchmark for molecular machine learning
Abstract
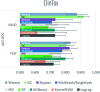
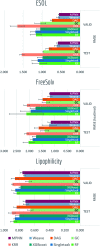
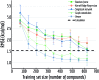
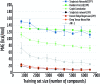
Molecular machine learning has been maturing rapidly over the last few years. Improved methods and the presence of larger datasets have enabled machine learning algorithms to make increasingly accurate predictions about molecular properties. However, algorithmic progress has been limited due to the lack of a standard benchmark to compare the efficacy of proposed methods; most new algorithms are benchmarked on different datasets making it challenging to gauge the quality of proposed methods. This work introduces MoleculeNet, a large scale benchmark for molecular machine learning. MoleculeNet curates multiple public datasets, establishes metrics for evaluation, and offers high quality open-source implementations of multiple previously proposed molecular featurization and learning algorithms (released as part of the DeepChem open source library). MoleculeNet benchmarks demonstrate that learnable representations are powerful tools for molecular machine learning and broadly offer the best performance. However, this result comes with caveats. Learnable representations still struggle to deal with complex tasks under data scarcity and highly imbalanced classification. For quantum mechanical and biophysical datasets, the use of physics-aware featurizations can be more important than choice of particular learning algorithm.
Figures







References
-
- Gasteiger J., Zupan J. Angew. Chem., Int. Ed. 1993;32:503–527.
-
- Zupan J. and Gasteiger J., Neural networks in chemistry and drug design, John Wiley & Sons, Inc., 1999.
-
- Varnek A., Baskin I. J. Chem. Inf. Model. 2012;52:1413–1437. - PubMed
-
- Devillers J., Neural networks in QSAR and drug design, Academic Press, 1996.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

