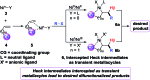
Ni-catalysed regioselective 1,2-diarylation of unactivated olefins by stabilizing Heck intermediates as pyridylsilyl-coordinated transient metallacycles
- PMID: 29629157
- PMCID: PMC5872806
- DOI: 10.1039/c7sc04351a
Ni-catalysed regioselective 1,2-diarylation of unactivated olefins by stabilizing Heck intermediates as pyridylsilyl-coordinated transient metallacycles
Abstract
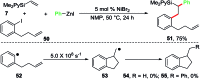
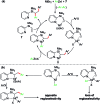
We report a Ni-catalysed diarylation of unactivated olefins in dimethylpyridylvinylsilane by intercepting Heck C(sp3)-NiX intermediates, derived from aryl halides, with arylzinc reagents. This approach utilizes a modifiable pyridylsilyl moiety as a coordinating group that plays a dual role of intercepting oxidative addition species to promote Heck carbometallation, and stabilizing the Heck C(sp3)-NiX intermediates as transient metallacycles to suppress β-hydride elimination, and facilitate transmetalation/reductive elimination. This method affords 1,2-diarylethylsilanes, which can be readily oxidized to 1,2-diarylethanols that occur as structural motifs in 3-aryl-3,4-dihydroisocoumarin and dihydrostilbenoid natural products.
Figures

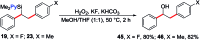

References
-
-
For dicarbofunctionalization of activated olefins by conjugate addition/enolate interception, see:
- Guo H.-C., Ma J.-A. Angew. Chem., Int. Ed. 2006;45:354. - PubMed
- Qin T., Cornella J., Li C., Malins L. R., Edwards J. T., Kawamura S., Maxwell B. D., Eastgate M. D., Baran P. S. Science. 2016;352:801. - PMC - PubMed
-
-
- Liao L., Jana R., Urkalan K. B., Sigman M. S. J. Am. Chem. Soc. 2011;133:5784. - PMC - PubMed
- Wu X., Lin H.-C., Li M.-L., Li L.-L., Han Z.-Y., Gong L.-Z. J. Am. Chem. Soc. 2015;137:13476. - PubMed
- Kuang Z., Yang K., Song Q. Org. Lett. 2017;19:2702. - PubMed
- Terao J., Nii S., Chowdhury F. A., Nakamura A., Kambe N. Adv. Synth. Catal. 2004;346:905.
- Mizutani K., Shinokubo H., Oshima K. Org. Lett. 2003;5:3959. - PubMed
-
-
For examples of two-component dicarbofunctionalizations of tethered olefins, see:
- Dhungana R. K., Shrestha B., Thapa-Magar R., Basnet P., Giri R. Org. Lett. 2017;19:2154. - PubMed
- Balme G., Bouyssi D., Lomberget T., Monteiro N. Synthesis. 2003;2003:2115.
- Fournet G., Balme G., Gore J. Tetrahedron Lett. 1987;28:4533.
- Kim J. G., Son Y. H., Seo J. W., Kang E. J. Eur. J. Org. Chem. 2015;2015:1781.
- Phapale V. B., Buñuel E., García-Iglesias M., Cárdenas D. J. Angew. Chem., Int. Ed. 2007;46:8790. - PubMed
- Nakamura M., Ito S., Matsuo K., Nakamura E. Synlett. 2005;2005:1794.
- Wakabayashi K., Yorimitsu H., Oshima K. J. Am. Chem. Soc. 2001;123:5374. - PubMed
- Yan C.-S., Peng Y., Xu X.-B., Wang Y.-W. Chem.–Eur. J. 2012;18:6039. - PubMed
- Seashore-Ludlow B., Somfai P. Org. Lett. 2012;14:3858. - PubMed
- Grigg R., Sansano J., Santhakumar V., Sridharan V., Thangavelanthum R., Thornton-Pett M., Wilson D. Tetrahedron. 1997;53:11803.
- Thapa S., Basnet P., Giri R. J. Am. Chem. Soc. 2017;139:5700. - PubMed
- You W., Brown M. K. J. Am. Chem. Soc. 2015;137:14578. - PubMed
- You W., Brown M. K. J. Am. Chem. Soc. 2014;136:14730. - PubMed
- Cong H., Fu G. C. J. Am. Chem. Soc. 2014;136:3788. - PMC - PubMed
- McMahon C. M., Renn M. S., Alexanian E. J. Org. Lett. 2016;18:4148. - PMC - PubMed
- Vaupel A., Knochel P. J. Org. Chem. 1996;61:5743. - PubMed
- Ishiyama T., Murata M., Suzuki A., Miyaura N. J. Chem. Soc., Chem. Commun. 1995:295.
- Walker J. A., Vickerman K. L., Humke J. N., Stanley L. M. J. Am. Chem. Soc. 2017;139:10228. - PubMed
-
-
- Saini V., Sigman M. S. J. Am. Chem. Soc. 2012;134:11372. - PMC - PubMed
- Werner E. W., Urkalan K. B., Sigman M. S. Org. Lett. 2010;12:2848. - PMC - PubMed
- Saini V., Liao L., Wang Q., Jana R., Sigman M. S. Org. Lett. 2013;15:5008. - PMC - PubMed
- Urkalan K. B., Sigman M. S. Angew. Chem., Int. Ed. 2009;48:3146. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

