Dye-sensitized electron transfer from TiO2 to oxidized triphenylamines that follows first-order kinetics
- PMID: 29629161
- PMCID: PMC5874694
- DOI: 10.1039/c7sc03839a
Dye-sensitized electron transfer from TiO2 to oxidized triphenylamines that follows first-order kinetics
Erratum in
-
Correction: Dye-sensitized electron transfer from TiO2 to oxidized triphenylamines that follows first-order kinetics.Chem Sci. 2018 Jan 26;9(6):1700. doi: 10.1039/c7sc90080e. eCollection 2018 Feb 14. Chem Sci. 2018. PMID: 30090315 Free PMC article.
Abstract
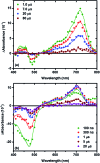
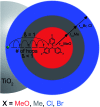
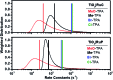
Two sensitizers, [Ru(bpy)2(dcb)]2+ (RuC) and [Ru(bpy)2(dpb)]2+ (RuP), where bpy is 2,2'-bipyridine, dcb is 4,4'-dicarboxylic acid-2,2'-bipyridine and dpb is 4,4'-diphosphonic acid-2,2'-bipyridine, were anchored to mesoporous TiO2 thin films and utilized to sensitize the reaction of TiO2 electrons with oxidized triphenylamines, TiO2(e-) + TPA+ → TiO2 + TPA, to visible light in CH3CN electrolytes. A family of four symmetrically substituted triphenylamines (TPAs) with formal Eo(TPA+/0) reduction potentials that spanned a 0.5 eV range was investigated. Surprisingly, the reaction followed first-order kinetics for two TPAs that provided the largest thermodynamic driving force. Such first-order reactivity indicates a strong Coulombic interaction between TPA+ and TiO2 that enables the injected electron to tunnel back in one concerted step. The kinetics for the other TPA derivatives were non-exponential and were modelled with the Kohlrausch-William-Watts (KWW) function. A Perrin-like reaction sphere model is proposed to rationalize the kinetic data. The activation energies were the same for all of the TPAs, within experimental error. The average rate constants were found to increase with the thermodynamic driving force, consistent with electron transfer in the Marcus normal region.
Figures





References
-
- Lewis N. S. J. Phys. Chem. B. 1998;102:4843–4855.
-
- Ardo S., Meyer G. J. Chem. Soc. Rev. 2009;38:115–164. - PubMed
-
- Hisatomi T., Kubota J., Domen K. Chem. Soc. Rev. 2014;43:7520–7535. - PubMed
-
- Zigler D. F., Morseth Z. A., Wang L., Ashford D. L., Brennaman M. K., Grumstrup E. M., Brigham E. C., Gish M. K., Dillon R. J., Alibabaei L., Meyer G. J., Meyer T. J., Papanikolas J. M. J. Am. Chem. Soc. 2016;138:4426–4438. - PubMed
-
- Listorti A., O’Regan B., Durrant J. R. Chem. Mater. 2011;23:3381–3399.
LinkOut - more resources
Full Text Sources
Other Literature Sources

