Stereoselective construction of sterically hindered oxaspirocycles via chiral bidentate directing group-mediated C(sp3)-O bond formation
- PMID: 29629170
- PMCID: PMC5875089
- DOI: 10.1039/c7sc04691j
Stereoselective construction of sterically hindered oxaspirocycles via chiral bidentate directing group-mediated C(sp3)-O bond formation
Abstract
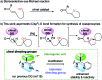
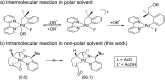
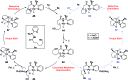
The systematic investigation of chiral bidentate auxiliaries has resulted in the discovery of a chiral 2,2-dimethyl-1-(pyridin-2-yl)propan-1-amine-derived directing group that enables stereoselective palladium(ii)-catalyzed intramolecular C(sp3)-O bond formation. This new chiral directing group exhibited high reactivity in the activation of methylene C(sp3)-H bonds with excellent levels of stereoselectivity (a diastereomeric ratio of up to 39 : 1), which allowed the construction of a wide range of oxaspirocycles. Mechanistic investigations were also conducted to elucidate the reaction mechanism and understand the origin of the diastereoselectivity. DFT calculations suggest that only modest levels of diastereoselectivity are accomplished at the rate-determining C-H metalation-deprotonation step and the d.r. is further enriched at the reductive elimination step.
Figures


Similar articles
-
2-(Pyridin-2-yl)isopropyl (PIP) Amine: An Enabling Directing Group for Divergent and Asymmetric Functionalization of Unactivated Methylene C(sp3)-H Bonds.Acc Chem Res. 2021 Jun 15;54(12):2750-2763. doi: 10.1021/acs.accounts.1c00168. Epub 2021 May 21. Acc Chem Res. 2021. PMID: 34019373
-
Experimental-Computational Synergy for Selective Pd(II)-Catalyzed C-H Activation of Aryl and Alkyl Groups.Acc Chem Res. 2017 Nov 21;50(11):2853-2860. doi: 10.1021/acs.accounts.7b00440. Epub 2017 Nov 8. Acc Chem Res. 2017. PMID: 29115826 Free PMC article.
-
Asymmetric C-H functionalization of cyclopropanes using an isoleucine-NH2 bidentate directing group.Chem Sci. 2015 Jun 1;6(6):3611-3616. doi: 10.1039/c5sc01137j. Epub 2015 Apr 16. Chem Sci. 2015. PMID: 29511524 Free PMC article.
-
Regio- and Stereoselective Functionalization Enabled by Bidentate Directing Groups.Chem Rec. 2021 Dec;21(12):3613-3627. doi: 10.1002/tcr.202100117. Epub 2021 Jun 4. Chem Rec. 2021. PMID: 34086390 Review.
-
The Emergence of Palladium-Catalyzed C(sp3 )-H Functionalization of Free Carboxylic Acids.Chem Asian J. 2021 Mar 1;16(5):397-408. doi: 10.1002/asia.202001440. Epub 2021 Feb 2. Chem Asian J. 2021. PMID: 33427411 Review.
Cited by
-
Total Synthesis of (-)-Maximiscin.J Am Chem Soc. 2020 May 13;142(19):8608-8613. doi: 10.1021/jacs.0c03202. Epub 2020 May 1. J Am Chem Soc. 2020. PMID: 32338003 Free PMC article.
-
Transition-Metal-Catalyzed Annulations Involving the Activation of C(sp3 )-H Bonds.Angew Chem Int Ed Engl. 2022 Feb 1;61(6):e202112848. doi: 10.1002/anie.202112848. Epub 2021 Dec 2. Angew Chem Int Ed Engl. 2022. PMID: 34699657 Free PMC article. Review.
-
Synthesis of 3,3-oxaspirocycles via cobalt-catalyzed cascade C-H activation and carboamidation of alkynes.Commun Chem. 2025 Jul 5;8(1):198. doi: 10.1038/s42004-025-01580-5. Commun Chem. 2025. PMID: 40617957 Free PMC article.
-
Oxa-spirocycles: synthesis, properties and applications.Chem Sci. 2021 Jul 27;12(34):11294-11305. doi: 10.1039/d1sc03615g. eCollection 2021 Sep 1. Chem Sci. 2021. PMID: 34667540 Free PMC article.
-
Palladium(0)-Catalyzed Directed syn-1,2-Carboboration and -Silylation: Alkene Scope, Applications in Dearomatization, and Stereocontrol by a Chiral Auxiliary.Angew Chem Int Ed Engl. 2019 Nov 18;58(47):17068-17073. doi: 10.1002/anie.201910304. Epub 2019 Oct 8. Angew Chem Int Ed Engl. 2019. PMID: 31538388 Free PMC article.
References
-
- Fukaya H., Hitotsuyanagi Y., Aoyagi Y., Shu Z., Komatsu K., Takeya K. Chem. Pharm. Bull. 2013;61:1085. - PubMed
- Zhang G., Wu G., Zhu T., Kurtn T., Mandi A., Jiao J., Li J., Qi X., Gu Q., Li D. J. Nat. Prod. 2013;76:1946. - PubMed
- Hirasawa Y., Morita H., Shiro M., Kobayashi J. Org. Lett. 2003;5:3991. - PubMed
- Aoki S., Watanabe Y., Sanagawa M., Setiawan A., Kotoku N., Kobayashi M. J. Am. Chem. Soc. 2006;128:3148. - PubMed
- Rosenberg S., Leino R. Tetrahedron Lett. 2009;50:5305.
- Jiao Z.-W., Zhang S.-Y., He C., Tu Y.-Q., Wang S.-H., Zhang F.-M., Zhang Y.-Q., Li H. Angew. Chem., Int. Ed. 2012;51:8811. - PubMed
-
- Bemis G. W., Murcko M. A. J. Med. Chem. 1996;39:2887. - PubMed
- Lipkus A. H., Yuan Q., Lucas K. A., Funk S. A., Bartelt W. F., Schenck R. J., Trippe A. J. J. Org. Chem. 2008;73:4443. - PubMed
- Lameijer E. W., Kok J. N., Back T., Ijzerman A. P. J. Chem. Inf. Model. 2006;46:553. - PubMed
- Burkhard J. A., Wagner B., Fischer H., Schuler F., Müller K., Carreira E. M. Angew. Chem., Int. Ed. 2010;49:3524. - PubMed
-
-
For selected examples of spirocycle synthesis, see:
- Teng X., Cefalo D. R., Schrock R. R., Hoveyda A. H. J. Am. Chem. Soc. 2002;124:10779. - PubMed
- Lejkowski M., Banerjee P., Runsink J., Gais H.-J. Org. Lett. 2008;10:2713. - PubMed
- Zhang Q.-W., Fan C.-A., Zhang H.-J., Tu Y.-Q., Zhao Y.-M., Gu P., Chen Z.-M. Angew. Chem., Int. Ed. 2009;48:8572. - PubMed
- Badillo J. J., Arevalo G. E., Fettinger J. C., Franz A. K. Org. Lett. 2011;13:418. - PubMed
- Jiao Z.-W., Zhang S.-Y., He C., Tu Y.-Q., Wang S.-H., Zhang F.-M., Zhang Y.-Q., Li H. Angew. Chem., Int. Ed. 2012;51:8811. - PubMed
- Burkhard J. A., Guerot C., Knust H., Carreira E. M. Org. Lett. 2012;14:66. - PubMed
- Fu J., Shen H., Chang Y., Li C., Gong J., Yang Z. Chem.–Eur. J. 2014;20:12881. - PubMed
- Rios R. Chem. Soc. Rev. 2012;41:1060. - PubMed
-
-
- Mitsunobu O., in Comprehensive Organic Synthesis ed. B. M. Trost, I. Fleming, Pergamon, Oxford, 1991, p. 1.
- Advanced Organic Chemistry ed. M. B. Smith, J. March, Wiley Interscience: New York, 2001, p. 479.
-
-
For recent reviews of asymmetric oxa-Michael reactions, see:
- Yang J. W., Hoffmann S., List B. Chem. Rev. 2007;107:5471. - PubMed
- Nising C. F., Bräse S. Chem. Soc. Rev. 2008;37:1218. - PubMed
- Moyano A., Rios R. Chem. Rev. 2011;111:4703. - PubMed
- Nising C. F., Bräse S. Chem. Soc. Rev. 2012;41:988. - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

