The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy
- PMID: 29630386
- PMCID: PMC6223160
- DOI: 10.1259/bjr.20170762
The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy
Abstract
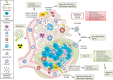
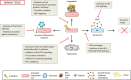
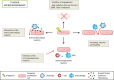
Altered by ionising radiation, the vascular network is considered as a prime target to limit normal tissue damage and improve tumour control in radiotherapy (RT). Irradiation damages and/or activates endothelial cells, which then participate in the recruitment of circulating cells, especially by overexpressing cell adhesion molecules, but also by other as yet unknown mechanisms. Radiation-induced lesions are associated with infiltration of immune-inflammatory cells from the blood and/or the lymph circulation. Damaged cells from the tissues and immune-inflammatory resident cells release factors that attract cells from the circulation, leading to the restoration of tissue balance by fighting against infection, elimination of damaged cells and healing of the injured area. In normal tissues that surround the tumours, the development of an immune-inflammatory reaction in response to radiation-induced tissue injury can turn out to be chronic and deleterious for the organ concerned, potentially leading to fibrosis and/or necrosis of the irradiated area. Similarly, tumours can elicit an immune-inflammation reaction, which can be initialised and amplified by cancer therapy such as radiotherapy, although immune checkpoints often allow many cancers to be protected by inhibiting the T-cell signal. Herein, we have explored the involvement of vascular endothelium in the fate of healthy tissues and tumours undergoing radiotherapy. This review also covers current investigations that take advantage of the radiation-induced response of the vasculature to spare healthy tissue and/or target tumours better.
Figures


References
-
- Shrieve DC, Loeffler JS. Human radiation injury. Philadelphia: The British Institute of Radiology.; 2011.
-
- Stone HB, McBride WH, Coleman CN. Modifying normal tissue damage postirradiation. Report of a workshop sponsored by the Radiation Research Program, National Cancer Institute, Bethesda, Maryland, September 6-8, 2000. Radiat Res 2002; 157: 204–23. - PubMed
-
- DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology: The British Institute of Radiology.; 2011.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

