Loss of biliverdin reductase-A promotes lipid accumulation and lipotoxicity in mouse proximal tubule cells
- PMID: 29631357
- PMCID: PMC6139518
- DOI: 10.1152/ajprenal.00495.2017
Loss of biliverdin reductase-A promotes lipid accumulation and lipotoxicity in mouse proximal tubule cells
Abstract
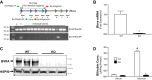
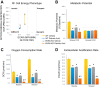
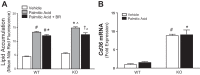
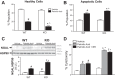
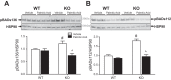
Obesity and increased lipid availability have been implicated in the development and progression of chronic kidney disease. One of the major sites of renal lipid accumulation is in the proximal tubule cells of the kidney, suggesting that these cells may be susceptible to lipotoxicity. We previously demonstrated that loss of hepatic biliverdin reductase A (BVRA) causes fat accumulation in livers of mice on a high-fat diet. To determine the role of BVRA in mouse proximal tubule cells, we generated a CRISPR targeting BVRA for a knockout in mouse proximal tubule cells (BVRA KO). The BVRA KO cells had significantly less metabolic potential and mitochondrial respiration, which was exacerbated by treatment with palmitic acid, a saturated fatty acid. The BVRA KO cells also showed increased intracellular triglycerides which were associated with higher fatty acid uptake gene cluster of differentiation 36 as well as increased de novo lipogenesis as measured by higher neutral lipids. Additionally, neutrophil gelatinase-associated lipocalin 1 expression, annexin-V FITC staining, and lactate dehydrogenase assays all demonstrated that BVRA KO cells are more sensitive to palmitic acid-induced lipotoxicity than wild-type cells. Phosphorylation of BAD which plays a role in cell survival pathways, was significantly reduced in palmitic acid-treated BVRA KO cells. These data demonstrate the protective role of BVRA in proximal tubule cells against saturated fatty acid-induced lipotoxicity and suggest that activating BVRA could provide a benefit in protecting from obesity-induced kidney injury.
Keywords: CRISPR; apoptosis; bilirubin; kidney; obesity; palmitic acid.
Figures

References
-
- Belo L, Nascimento H, Kohlova M, Bronze-da-Rocha E, Fernandes J, Costa E, Catarino C, Aires L, Mansilha HF, Rocha-Pereira P, Quintanilha A, Rêgo C, Santos-Silva A. Body fat percentage is a major determinant of total bilirubin independently of UGT1A1*28 polymorphism in young obese. PLoS One 9: e98467, 2014. doi: 10.1371/journal.pone.0098467. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

