Elastin, arterial mechanics, and cardiovascular disease
- PMID: 29631368
- PMCID: PMC6139627
- DOI: 10.1152/ajpheart.00087.2018
Elastin, arterial mechanics, and cardiovascular disease
Abstract
Large, elastic arteries are composed of cells and a specialized extracellular matrix that provides reversible elasticity and strength. Elastin is the matrix protein responsible for this reversible elasticity that reduces the workload on the heart and dampens pulsatile flow in distal arteries. Here, we summarize the elastin protein biochemistry, self-association behavior, cross-linking process, and multistep elastic fiber assembly that provide large arteries with their unique mechanical properties. We present measures of passive arterial mechanics that depend on elastic fiber amounts and integrity such as the Windkessel effect, structural and material stiffness, and energy storage. We discuss supravalvular aortic stenosis and autosomal dominant cutis laxa-1, which are genetic disorders caused by mutations in the elastin gene. We present mouse models of supravalvular aortic stenosis, autosomal dominant cutis laxa-1, and graded elastin amounts that have been invaluable for understanding the role of elastin in arterial mechanics and cardiovascular disease. We summarize acquired diseases associated with elastic fiber defects, including hypertension and arterial stiffness, diabetes, obesity, atherosclerosis, calcification, and aneurysms and dissections. We mention animal models that have helped delineate the role of elastic fiber defects in these acquired diseases. We briefly summarize challenges and recent advances in generating functional elastic fibers in tissue-engineered arteries. We conclude with suggestions for future research and opportunities for therapeutic intervention in genetic and acquired elastinopathies.
Keywords: aorta; compliance; elasticity; extracellular matrix; stiffness.
Figures

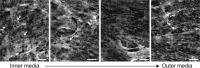


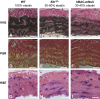
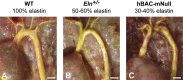

References
-
- Akima T, Nakanishi K, Suzuki K, Katayama M, Ohsuzu F, Kawai T. Soluble elastin decreases in the progress of atheroma formation in human aorta. Circ J 73: 2154–2162, 2009. - PubMed
-
- Alessi MC, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, Geel O, Juhan-Vague I. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 49: 1374–1380, 2000. doi: 10.2337/diabetes.49.8.1374. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

