Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports
- PMID: 29631541
- PMCID: PMC5891905
- DOI: 10.1186/s12879-018-3043-7
Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports
Abstract
Background: Nontuberculous Mycobacteria (NTM) are environmental microorganisms that can affect human health. A 2009-2010 occurrence survey of NTM in potable tap water samples indicated an increased recovery rate for many clinically significant species such as M. avium (30%) and M. abscessus (12%). To determine if these trends by species were mirrored in human infections, isolation rates of NTM species identified in clinical laboratory reports from four states were evaluated.
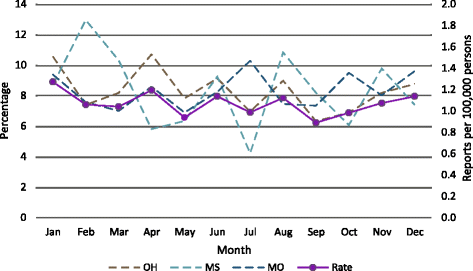
Method: Clinical laboratory reports from the Mississippi, Missouri, Ohio, and Wisconsin Health Departments were used to investigate the species of NTM isolated from human specimens in 2014. The NTM positive specimen reports were tabulated for each species and complex/group. The number of reports by month were used to investigate seasonal trends. The 2014 isolation rates were compared to historic values to examine longitudinal trends.
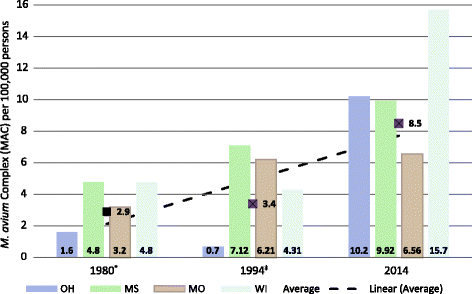
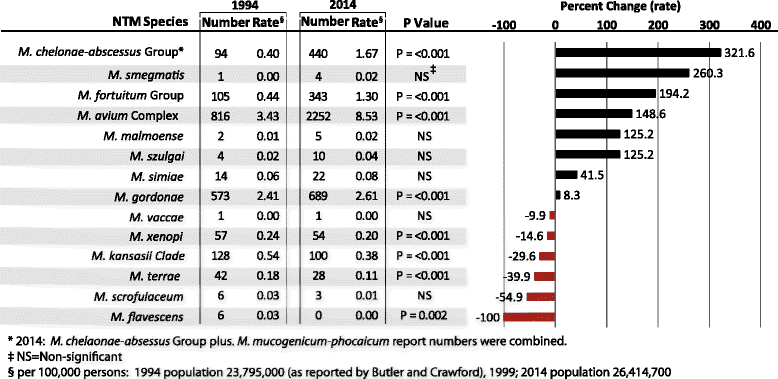
Results: The positive rate of NTM specimens increased from 8.2 per 100,000 persons in 1994 to 16 per 100,000 persons in 2014 (or 13.3 per 100,000 after excluding Mycobacterium gordonae). Changes in NTM diversity were observed in complex/groups known to be clinically significant. Between 1994 and 2014 the rate implicating M. abscesses-chelonae group and M. avium complex increased by 322 and 149%, respectively.
Conclusions: Based on public health data supplied by the four State's Health Departments and the 2014 U.S. population, 50,976 positive NTM specimen reports per year were projected for the nation; serving as an indicator for the national potential disease burden that year.
Keywords: Clinical laboratory reports; Epidemiology; Isolation rates; Nontuberculous mycobacteria (NTM); Report rates; Seasonal; Species; United States.
Conflict of interest statement
Ethics approval and consent to participate
This study was determined to be exempt from Institution review board review by United States environmental protection agency.
Consent for publication
Not applicable.
Competing interests
The author declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970–976. doi: 10.1164/rccm.201002-0310OC. - DOI - PMC - PubMed
-
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. - DOI - PubMed
-
- Olivier KN, Weber DJ, Lee JH, Handler A, Tudor G, Molina PL, Tomashefski J, Knowles MR, Nontuberculous Mycobacteria in Cystic Fibrosis Study G Nontuberculous mycobacteria. II: nested-cohort study of impact on cystic fibrosis lung disease. Am J Respir Crit Care Med. 2003;167(6):835–840. doi: 10.1164/rccm.200207-679OC. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

