High Levels of Intrinsic Tetracycline Resistance in Mycobacterium abscessus Are Conferred by a Tetracycline-Modifying Monooxygenase
- PMID: 29632012
- PMCID: PMC5971581
- DOI: 10.1128/AAC.00119-18
High Levels of Intrinsic Tetracycline Resistance in Mycobacterium abscessus Are Conferred by a Tetracycline-Modifying Monooxygenase
Abstract
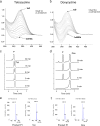
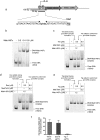
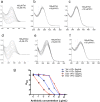
Tetracyclines have been one of the most successful classes of antibiotics. However, its extensive use has led to the emergence of widespread drug resistance, resulting in discontinuation of use against several bacterial infections. Prominent resistance mechanisms include drug efflux and the use of ribosome protection proteins. Infrequently, tetracyclines can be inactivated by the TetX class of enzymes, also referred to as tetracycline destructases. Low levels of tolerance to tetracycline in Mycobacterium smegmatis and Mycobacterium tuberculosis have been previously attributed to the WhiB7-dependent TetV/Tap efflux pump. However, Mycobacterium abscessus is ∼500-fold more resistant to tetracycline than M. smegmatis and M. tuberculosis In this report, we show that this high level of resistance to tetracycline and doxycycline in M. abscessus is conferred by a WhiB7-independent tetracycline-inactivating monooxygenase, MabTetX (MAB_1496c). The presence of sublethal doses of tetracycline and doxycycline results in a >200-fold induction of MabTetX, and an isogenic deletion strain is highly sensitive to both antibiotics. Further, purified MabTetX can rapidly monooxygenate both antibiotics. We also demonstrate that expression of MabTetX is repressed by MabTetRx, by binding to an inverted repeat sequence upstream of MabTetRx; the presence of either antibiotic relieves this repression. Moreover, anhydrotetracycline (ATc) can effectively inhibit MabTetX activity in vitro and decreases the MICs of both tetracycline and doxycycline in vivo Finally, we show that tigecycline, a glycylcycline tetracycline, not only is a poor substrate of MabTetX but also is incapable of inducing the expression of MabTetX. This is therefore the first demonstration of a tetracycline-inactivating enzyme in mycobacteria. It (i) elucidates the mechanism of tetracycline resistance in M. abscessus, (ii) demonstrates the use of an inhibitor that can potentially reclaim the use of tetracycline and doxycycline, and (iii) identifies two sequential bottlenecks-MabTetX and MabTetRx-for acquiring resistance to tigecycline, thereby reiterating its use against M. abscessus.
Keywords: M. abscessus; antibiotic resistance; drug inactivation; intrinsic resistance; tetracyclines.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

