Matrix Metalloproteinase-Mediated Blood-Brain Barrier Dysfunction in Epilepsy
- PMID: 29632167
- PMCID: PMC5932641
- DOI: 10.1523/JNEUROSCI.2751-17.2018
Matrix Metalloproteinase-Mediated Blood-Brain Barrier Dysfunction in Epilepsy
Abstract
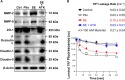
The blood-brain barrier is dysfunctional in epilepsy, thereby contributing to seizure genesis and resistance to antiseizure drugs. Previously, several groups reported that seizures increase brain glutamate levels, which leads to barrier dysfunction. One critical component of barrier dysfunction is brain capillary leakage. Based on our preliminary data, we hypothesized that glutamate released during seizures mediates an increase in matrix-metalloproteinase (MMP) expression and activity levels, thereby contributing to barrier leakage. To test this hypothesis, we exposed isolated brain capillaries from male Sprague Dawley rats to glutamate ex vivo and used an in vivo/ex vivo approach of isolated brain capillaries from female Wistar rats that experienced status epilepticus as an acute seizure model. We found that exposing isolated rat brain capillaries to glutamate increased MMP-2 and MMP-9 protein and activity levels, and decreased tight junction protein levels, which resulted in barrier leakage. We confirmed these findings in vivo in rats after status epilepticus and in brain capillaries from male mice lacking cytosolic phospholipase A2 Together, our data support the hypothesis that glutamate released during seizures signals an increase in MMP-2 and MMP-9 protein expression and activity levels, resulting in blood-brain barrier leakage.SIGNIFICANCE STATEMENT The mechanism leading to seizure-mediated blood-brain barrier dysfunction in epilepsy is poorly understood. In the present study, we focused on defining this mechanism in the brain capillary endothelium. We demonstrate that seizures trigger a pathway that involves glutamate signaling through cytosolic phospholipase A2, which increases MMP levels and decreases tight junction protein expression levels, resulting in barrier leakage. These findings may provide potential therapeutic avenues within the blood-brain barrier to limit barrier dysfunction in epilepsy and decrease seizure burden.
Keywords: Blood-Brain barrier; MMP; barrier dysfunction; barrier leakage; cPLA2.
Copyright © 2018 the authors 0270-6474/18/384301-15$15.00/0.
Figures


Comment in
-
cPLA2 in Epilepsy: Shutting Down the Leaker at Its Source.Epilepsy Curr. 2018 Sep-Oct;18(5):334-335. doi: 10.5698/1535-7597.18.5.334. Epilepsy Curr. 2018. PMID: 30464739 Free PMC article. No abstract available.
References
-
- Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, El-Zammar Z, Alam S, Hallenbeck JM, Kidwell CS, Warach S (2010) Blood-Brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke 41:e123–128. 10.1161/STROKEAHA.109.570515 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
