Human hydroxymethylbilane synthase: Molecular dynamics of the pyrrole chain elongation identifies step-specific residues that cause AIP
- PMID: 29632172
- PMCID: PMC5924904
- DOI: 10.1073/pnas.1719267115
Human hydroxymethylbilane synthase: Molecular dynamics of the pyrrole chain elongation identifies step-specific residues that cause AIP
Abstract
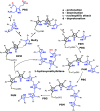
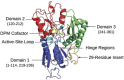
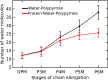
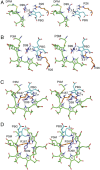
Hydroxymethylbilane synthase (HMBS), the third enzyme in the heme biosynthetic pathway, catalyzes the head-to-tail condensation of four molecules of porphobilinogen (PBG) to form the linear tetrapyrrole 1-hydroxymethylbilane (HMB). Mutations in human HMBS (hHMBS) cause acute intermittent porphyria (AIP), an autosomal-dominant disorder characterized by life-threatening neurovisceral attacks. Although the 3D structure of hHMBS has been reported, the mechanism of the stepwise polymerization of four PBG molecules to form HMB remains unknown. Moreover, the specific roles of each of the critical active-site residues in the stepwise enzymatic mechanism and the dynamic behavior of hHMBS during catalysis have not been investigated. Here, we report atomistic studies of HMB stepwise synthesis by using molecular dynamics (MD) simulations, mutagenesis, and in vitro expression analyses. These studies revealed that the hHMBS active-site loop movement and cofactor turn created space for the elongating pyrrole chain. Twenty-seven residues around the active site and water molecules interacted to stabilize the large, negatively charged, elongating polypyrrole. Mutagenesis of these active-site residues altered the binding site, hindered cofactor binding, decreased catalysis, impaired ligand exit, and/or destabilized the enzyme. Based on intermediate stages of chain elongation, R26 and R167 were the strongest candidates for proton transfer to deaminate the incoming PBG molecules. Unbiased random acceleration MD simulations identified R167 as a gatekeeper and facilitator of HMB egress through the space between the enzyme's domains and the active-site loop. These studies identified the specific active-site residues involved in each step of pyrrole elongation, thereby providing the molecular bases of the active-site mutations causing AIP.
Keywords: enzyme catalysis; molecular dynamics; structural biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Anderson KE, Sassa S, Bishop DF, Desnick RJ. 2001 Disorders of heme biosynthesis: X-linked sideroblastic anemia and the porphyrias. The Metabolic and Molecular Bases of Inherited Disease, eds Valle D, et al. (McGraw-Hill, New York), Chap 124. Available at ommbid.mhmedical.com/content.aspx?bookid=971&Sectionid=62638866.
-
- Bogorad L. The enzymatic synthesis of porphyrins from porphobilinogen. I. Uroporphyrin I. J Biol Chem. 1958;233:501–509. - PubMed
-
- Anderson PM, Desnick RJ. Purification and properties of uroporphyrinogen I synthase from human erythrocytes. Identification of stable enzyme-substrate intermediates. J Biol Chem. 1980;255:1993–1999. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

