Controlling compartmentalization by non-membrane-bound organelles
- PMID: 29632271
- PMCID: PMC5904305
- DOI: 10.1098/rstb.2017.0193
Controlling compartmentalization by non-membrane-bound organelles
Abstract
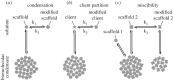
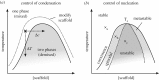
Compartmentalization is a characterizing feature of complexity in cells, used to organize their biochemistry. Membrane-bound organelles are most widely known, but non-membrane-bound liquid organelles also exist. These have recently been shown to form by phase separation of specific types of proteins known as scaffolds. This forms two phases: a condensate that is enriched in scaffold protein separated by a phase boundary from the cytoplasm or nucleoplasm with a low concentration of the scaffold protein. Phase separation is well known for synthetic polymers, but also appears important in cells. Here, we review the properties of proteins important for forming these non-membrane-bound organelles, focusing on the energetically favourable interactions that drive condensation. On this basis we make qualitative predictions about how cells may control compartmentalization by condensates; the partition of specific molecules to a condensate; the control of condensation and dissolution of condensates; and the regulation of condensate nucleation. There are emerging data supporting many of these predictions, although future results may prove incorrect. It appears that many molecules may have the ability to modulate condensate formation, making condensates a potential target for future therapeutics. The emerging properties of condensates are fundamentally unlike the properties of membrane-bound organelles. They have the capacity to rapidly integrate cellular events and act as a new class of sensors for internal and external environments.This article is part of the theme issue 'Self-organization in cell biology'.
Keywords: biomolecular condensates; cell compartmentalization; liquid–liquid phase separation; non-membrane-bound organelle.
© 2018 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
