A cynomolgus macaque model for Crimean-Congo haemorrhagic fever
- PMID: 29632370
- PMCID: PMC6717652
- DOI: 10.1038/s41564-018-0141-7
A cynomolgus macaque model for Crimean-Congo haemorrhagic fever
Abstract
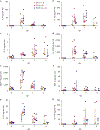
Crimean-Congo haemorrhagic fever (CCHF) is the most medically significant tick-borne disease, being widespread in the Middle East, Asia, Africa and parts of Europe 1 . Increasing case numbers, westerly movement and broadly ranging case fatality rates substantiate the concern of CCHF as a public health threat. Ixodid ticks of the genus Hyalomma are the vector for CCHF virus (CCHFV), an arbovirus in the genus Orthonairovirus of the family Nairoviridae. CCHFV naturally infects numerous wild and domestic animals via tick bite without causing obvious disease2,3. Severe disease occurs only in humans and transmission usually happens through tick bite or contact with infected animals or humans. The only CCHF disease model is a subset of immunocompromised mice4-6. Here, we show that following CCHFV infection, cynomolgus macaques exhibited hallmark signs of human CCHF with remarkably similar viral dissemination, organ pathology and disease progression. Histopathology showed infection of hepatocytes, endothelial cells and monocytes and fatal outcome seemed associated with endothelial dysfunction manifesting in a clinical shock syndrome with coagulopathy. This non-human primate model will be an invaluable asset for CCHFV countermeasures development.
Figures


References
-
- Bente DA et al. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 100, 159–189 (2013). - PubMed
-
- Hoogstraal H The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 15, 307–417 (1979). - PubMed
-
- Bereczky S et al. Crimean-Congo hemorrhagic fever virus infection is lethal for adult type I interferon receptor-knockout mice. J. Gen. Virol. 91, 1473–1477 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

