Flow cytometry analysis of Clostridium beijerinckii NRRL B-598 populations exhibiting different phenotypes induced by changes in cultivation conditions
- PMID: 29632557
- PMCID: PMC5887253
- DOI: 10.1186/s13068-018-1096-x
Flow cytometry analysis of Clostridium beijerinckii NRRL B-598 populations exhibiting different phenotypes induced by changes in cultivation conditions
Abstract
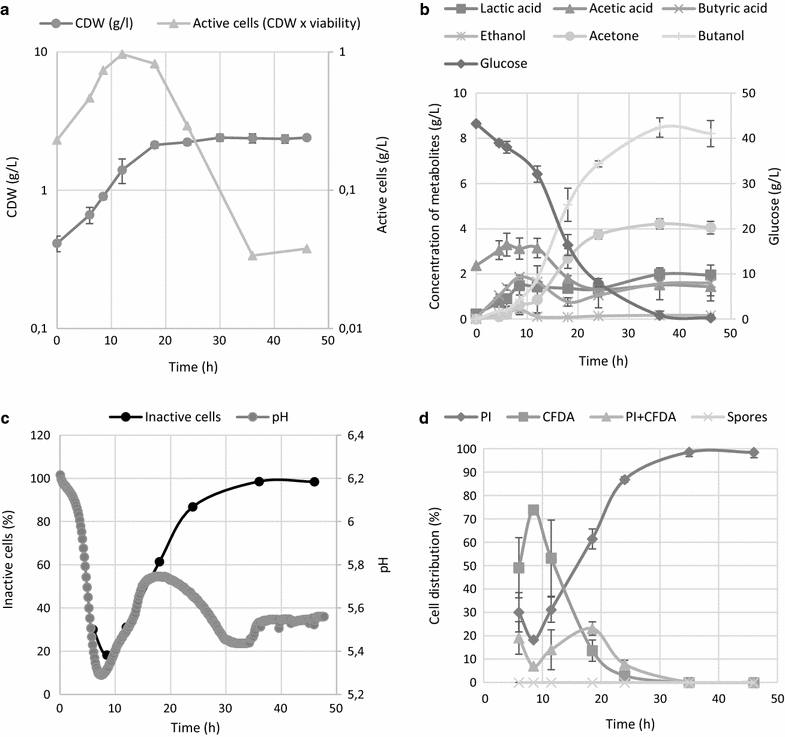
Background: Biobutanol production by clostridia via the acetone-butanol-ethanol (ABE) pathway is a promising future technology in bioenergetics , but identifying key regulatory mechanisms for this pathway is essential in order to construct industrially relevant strains with high tolerance and productivity. We have applied flow cytometric analysis to C. beijerinckii NRRL B-598 and carried out comparative screening of physiological changes in terms of viability under different cultivation conditions to determine its dependence on particular stages of the life cycle and the concentration of butanol.
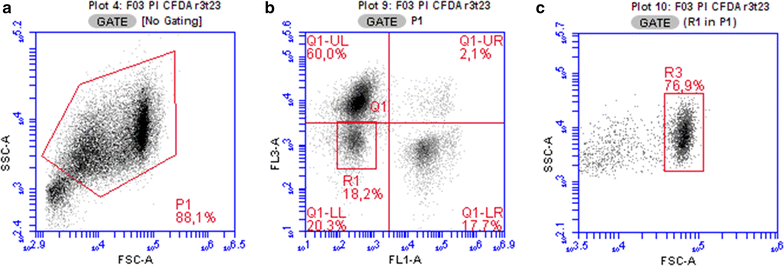
Results: Dual staining by propidium iodide (PI) and carboxyfluorescein diacetate (CFDA) provided separation of cells into four subpopulations with different abilities to take up PI and cleave CFDA, reflecting different physiological states. The development of a staining pattern during ABE fermentation showed an apparent decline in viability, starting at the pH shift and onset of solventogenesis, although an appreciable proportion of cells continued to proliferate. This was observed for sporulating as well as non-sporulating phenotypes at low solvent concentrations, suggesting that the increase in percentage of inactive cells was not a result of solvent toxicity or a transition from vegetative to sporulating stages. Additionally, the sporulating phenotype was challenged with butanol and cultivation with a lower starting pH was performed; in both these experiments similar trends were obtained-viability declined after the pH breakpoint, independent of the actual butanol concentration in the medium. Production characteristics of both sporulating and non-sporulating phenotypes were comparable, showing that in C. beijerinckii NRRL B-598, solventogenesis was not conditional on sporulation.
Conclusion: We have shown that the decline in C. beijerinckii NRRL B-598 culture viability during ABE fermentation was not only the result of accumulated toxic metabolites, but might also be associated with a special survival strategy triggered by pH change.
Keywords: ABE fermentation; Butanol; Clostridium; Cytometry; Fluorescence staining; Sporulation; Stress; Viability.
Figures



References
-
- Yusri IM, Mamat R, Najafi G, Razman A, Awad OI, Azmi WH, Ishak WFW, Shaiful AIM. Alcohol based automotive fuels from first four alcohol family in compression and spark ignition engine: a review on engine performance and exhaust emissions. Renew Sustain Energy Rev. 2017;77:169–181. doi: 10.1016/j.rser.2017.03.080. - DOI
-
- Pfromm PH, Amanor-Boadu V, Nelson R, Vadlani P, Madl R. Bio-butanol vs. bio-ethanol: a technical and economic assessment for corn and switchgrass fermented by yeast or Clostridium acetobutylicum. Biomass Bioenergy. 2010;34:515–524. doi: 10.1016/j.biombioe.2009.12.017. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

