Serpin functions in host-pathogen interactions
- PMID: 29632742
- PMCID: PMC5889911
- DOI: 10.7717/peerj.4557
Serpin functions in host-pathogen interactions
Abstract
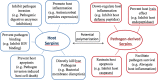
Serpins are a broadly distributed superfamily of protease inhibitors that are present in all kingdoms of life. The acronym, serpin, is derived from their function as potent serine proteases inhibitors. Early studies of serpins focused on their functions in haemostasis since modulating serine proteases activities are essential for coagulation. Additional research has revealed that serpins function in infection and inflammation, by modulating serine and cysteine proteases activities. The aim of this review is to summarize the accumulating findings and current understanding of the functions of serpins in host-pathogen interactions, serving as host defense proteins as well as pathogenic factors. We also discuss the potential crosstalk between host and pathogen serpins. We anticipate that future research will elucidate the therapeutic value of this novel target.
Keywords: Host-pathogen interaction; Infection; Inflammation; Serine protease; Serpin.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


References
-
- Abraham EG, Pinto SB, Ghosh A, Vanlandingham DL, Budd A, Higgs S, Kafatos FC, Jacobs-Lorena M, Michel K. An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16327–16332. doi: 10.1073/pnas.0508335102. - DOI - PMC - PubMed
-
- Ambadapadi S, Munuswamy-Ramanujam G, Zheng D, Sullivan C, Dai E, Morshed S, McFadden B, Feldman E, Pinard M, McKenna R, Tibbetts S, Lucas A. Reactive Center Loop (RCL) peptides derived from serpins display independent coagulation and immune modulating activities. Journal of Biological Chemistry. 2016;291:2874–2887. doi: 10.1074/jbc.M115.704841. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

