Late-Onset Inflammatory Response to Hyaluronic Acid Dermal Fillers
- PMID: 29632758
- PMCID: PMC5889432
- DOI: 10.1097/GOX.0000000000001532
Late-Onset Inflammatory Response to Hyaluronic Acid Dermal Fillers
Abstract
Objective: Even though injectable hyaluronic acid (HA)-based fillers are considered safe, rare complications, such as late-onset inflammatory reactions have been reported. Possible causes and effective treatments have not been formally described, so this work aims to discuss these and offer a formal protocol for treatment.
Methods: This article presents 5 clinical cases of late-onset inflammatory response occurring at least 3 months after uneventful injection of HA dermal filler.
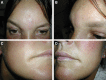
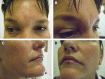
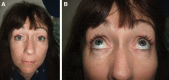
Results: Inflammation appeared spontaneously, usually 4-5 months after the last injection, but in 1 patient, almost 14 months later. One patient was injected at the same time with fillers manufactured by 2 different technologies. In this case, all areas treated with the same filler showed diffuse swelling of inflammatory nature, whereas the lips, treated with the second filler brand, remained unaffected. Four patients reported a flu-like illness or gastrointestinal upset a few days before the onset of dermal filler inflammation.
Conclusion: Late-onset inflammatory reactions to HA fillers may be self-limiting but are easily and rapidly treatable with oral steroids, and with hyaluronidase in the case of lumps. It is likely these reactions are due to a Type IV delayed hypersensitivity response. Delayed inflammation associated with HA fillers is nonbrand specific. However, the case where 2 different brands were injected during the same session, but only 1 brand triggered a hypersensitivity reaction, suggests that the technology used in the manufacturing process, and the subsequent differing products of degradation, may have an influence on potential allergic reactions to HA fillers.
Figures

References
-
- Surgery ASoAP. Cosmetic surgery national data bank statistics. 2015. Available at http://www.surgery.org/media/statistics. Accessed July 27, 2015. - PubMed
-
- Ozturk CN, Li Y, Tung R, et al. Complications following injection of soft-tissue fillers. Aesthet Surg J. 2013;33:862–877.. - PubMed
-
- Alijotas-Reig J, Fernández-Figueras MT, Puig L. Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin Arthritis Rheum. 2013;43:241–258.. - PubMed
-
- DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33:561–575.. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
