Association of human mitochondrial lysyl-tRNA synthetase with HIV-1 GagPol does not require other viral proteins
- PMID: 29632838
- PMCID: PMC5889485
- DOI: 10.1016/j.biopen.2016.02.004
Association of human mitochondrial lysyl-tRNA synthetase with HIV-1 GagPol does not require other viral proteins
Abstract
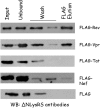
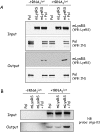
In human, the cytoplasmic (cLysRS) and mitochondrial (mLysRS) species of lysyl-tRNA synthetase are encoded by a single gene. Following HIV-1 infection, mLysRS is selectively taken up into viral particles along with the three tRNALys isoacceptors. The GagPol polyprotein precursor is involved in this process. With the aim to reconstitute in vitro the HIV-1 tRNA3Lys packaging complex, we first searched for the putative involvement of another viral protein in the selective viral hijacking of mLysRS only. After screening all the viral proteins, we observed that Vpr and Rev have the potential to interact with mLysRS, but that this association does not take place at the level of the assembly of mLysRS into the packaging complex. We also show that tRNA3Lys can form a ternary complex with the two purified proteins mLysRS and the Pol domain of GagPol, which mimicks its packaging complex.
Keywords: HIV-1; Lysyl-tRNA synthetase; Packaging complex; tRNA3Lys.
Figures


References
-
- Abbink T.E., Berkhout B. HIV-1 reverse transcription initiation: a potential target for novel antivirals? Virus Res. 2008;134:4–18. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

