Metabolic Engineering of the Shikimate Pathway for Production of Aromatics and Derived Compounds-Present and Future Strain Construction Strategies
- PMID: 29632862
- PMCID: PMC5879953
- DOI: 10.3389/fbioe.2018.00032
Metabolic Engineering of the Shikimate Pathway for Production of Aromatics and Derived Compounds-Present and Future Strain Construction Strategies
Abstract
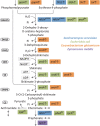
The aromatic nature of shikimate pathway intermediates gives rise to a wealth of potential bio-replacements for commonly fossil fuel-derived aromatics, as well as naturally produced secondary metabolites. Through metabolic engineering, the abundance of certain intermediates may be increased, while draining flux from other branches off the pathway. Often targets for genetic engineering lie beyond the shikimate pathway, altering flux deep in central metabolism. This has been extensively used to develop microbial production systems for a variety of compounds valuable in chemical industry, including aromatic and non-aromatic acids like muconic acid, para-hydroxybenzoic acid, and para-coumaric acid, as well as aminobenzoic acids and aromatic α-amino acids. Further, many natural products and secondary metabolites that are valuable in food- and pharma-industry are formed outgoing from shikimate pathway intermediates. (Re)construction of such routes has been shown by de novo production of resveratrol, reticuline, opioids, and vanillin. In this review, strain construction strategies are compared across organisms and put into perspective with requirements by industry for commercial viability. Focus is put on enhancing flux to and through shikimate pathway, and engineering strategies are assessed in order to provide a guideline for future optimizations.
Keywords: Shikimate pathway; aromatics; metabolic engineering; metabolic modelling; strain construction.
Figures

References
-
- Averesch N. J. H. (2016). Engineering Yeast Shikimate Pathway Towards Production of Aromatics: Rational Design of a Chassis Cell Using Systems and Synthetic Biology Ph.D. thesis, The University of Queensland, Brisbane.
-
- Averesch N. J. H., Kayser O. (2014). Assessing heterologous expression of hyoscyamine 6β-hydroxylase – a feasibility study. Procedia Chem. 13, 69–78. 10.1016/j.proche.2014.12.008 - DOI
-
- Averesch N. J. H., Martínez V. S., Nielsen L. K., Krömer J. O. (2018). Towards synthetic biology strategies for adipic acid production – an in-silico tool for combined thermodynamics and stoichiometric analysis of metabolic networks. ACS Synth. Biol. 7, 490–509. 10.1021/acssynbio.7b00304 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

