Structural and stereochemical diversity in prenylated indole alkaloids containing the bicyclo[2.2.2]diazaoctane ring system from marine and terrestrial fungi
- PMID: 29632911
- PMCID: PMC6102066
- DOI: 10.1039/c7np00042a
Structural and stereochemical diversity in prenylated indole alkaloids containing the bicyclo[2.2.2]diazaoctane ring system from marine and terrestrial fungi
Abstract
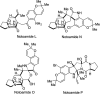
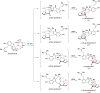
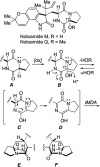
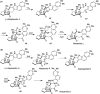
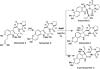
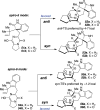
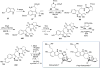
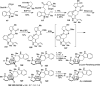
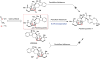
Covering: up to February 2017 Various fungi of the genera Aspergillus, Penicillium, and Malbranchea produce prenylated indole alkaloids possessing a bicyclo[2.2.2]diazaoctane ring system. After the discovery of distinct enantiomers of the natural alkaloids stephacidin A and notoamide B, from A. protuberus MF297-2 and A. amoenus NRRL 35660, another fungi, A. taichungensis, was found to produce their diastereomers, 6-epi-stephacidin A and versicolamide B, as major metabolites. Distinct enantiomers of stephacidin A and 6-epi-stephacidin A may be derived from a common precursor, notoamide S, by enzymes that form a bicyclo[2.2.2]diazaoctane core via a putative intramolecular hetero-Diels-Alder cycloaddition. This review provides our current understanding of the structural and stereochemical homologies and disparities of these alkaloids. Through the deployment of biomimetic syntheses, whole-genome sequencing, and biochemical studies, a unified biogenesis of both the dioxopiperazine and the monooxopiperazine families of prenylated indole alkaloids constituted of bicyclo[2.2.2]diazaoctane ring systems is presented.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures



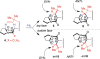

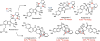







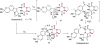



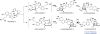



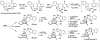



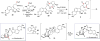
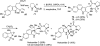




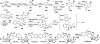


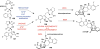
Similar articles
-
Taichunamides: Prenylated Indole Alkaloids from Aspergillus taichungensis (IBT 19404).Angew Chem Int Ed Engl. 2016 Jan 18;55(3):1128-32. doi: 10.1002/anie.201509462. Epub 2015 Dec 8. Angew Chem Int Ed Engl. 2016. PMID: 26644336 Free PMC article.
-
Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp.J Am Chem Soc. 2010 Sep 15;132(36):12733-40. doi: 10.1021/ja1049302. J Am Chem Soc. 2010. PMID: 20722388 Free PMC article.
-
Biomimetic and Concise Total Syntheses of Prenylated and Bicyclo[2.2.2]diazaoctane-Containing Indole Alkaloids Including Taichunamide A, Notoamide N and Versicolamide B.J Org Chem. 2024 Sep 6;89(17):12639-12650. doi: 10.1021/acs.joc.4c01559. Epub 2024 Aug 23. J Org Chem. 2024. PMID: 39180143
-
The Mutually Inspiring Biological and Chemical Synthesis of Fungal Bicyclo[2.2.2]diazaoctane Indole Alkaloids.Chem Rev. 2025 Feb 26;125(4):1718-1804. doi: 10.1021/acs.chemrev.4c00250. Epub 2025 Feb 10. Chem Rev. 2025. PMID: 39927617 Free PMC article. Review.
-
Fungal origins of the bicyclo[2.2.2]diazaoctane ring system of prenylated indole alkaloids.J Nat Prod. 2012 Apr 27;75(4):812-33. doi: 10.1021/np200954v. Epub 2012 Apr 15. J Nat Prod. 2012. PMID: 22502590 Free PMC article. Review.
Cited by
-
Combined Toxicity of the Most Common Indoor Aspergilli.Pathogens. 2023 Mar 14;12(3):459. doi: 10.3390/pathogens12030459. Pathogens. 2023. PMID: 36986381 Free PMC article.
-
Aspertaichamide B, a new anti-tumor prenylated indole alkaloid from the fungus Aspergillus japonicus TE-739D.Appl Microbiol Biotechnol. 2024 Sep 25;108(1):473. doi: 10.1007/s00253-024-13313-0. Appl Microbiol Biotechnol. 2024. PMID: 39320549 Free PMC article.
-
Unified total synthesis of the brevianamide alkaloids enabled by chemical investigations into their biosynthesis.Chem Sci. 2021 Dec 29;13(5):1313-1322. doi: 10.1039/d1sc05801k. eCollection 2022 Feb 2. Chem Sci. 2021. PMID: 35222915 Free PMC article.
-
Notoamide-type alkaloid induced apoptosis and autophagy via a P38/JNK signaling pathway in hepatocellular carcinoma cells.RSC Adv. 2019 Jun 25;9(34):19855-19868. doi: 10.1039/c9ra03640g. eCollection 2019 Jun 19. RSC Adv. 2019. PMID: 35519412 Free PMC article.
-
Diprenylated cyclodipeptide production by changing the prenylation sequence of the nature's synthetic machinery.Appl Microbiol Biotechnol. 2023 Jan;107(1):261-271. doi: 10.1007/s00253-022-12303-4. Epub 2022 Nov 28. Appl Microbiol Biotechnol. 2023. PMID: 36441211 Free PMC article.
References
-
- Birch AJ, Wright JJ. Tetrahedron Lett. 1970;26:2329–2344. - PubMed
-
- Birch AJ, Russel RA. Tetrahedron Lett. 1972;28:2999–3008.
-
- Steyn PS. Tetrahedron Lett. 1973;29:107–120.
-
- Birch AJ, Wright JJ. Chem. Commun. 1969:644–645.
-
- Paterson RRM, Hawksworth DL. Trans. Br. Mycol. Soc. 1985;85:95–100.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

