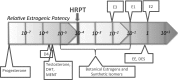
Human-relevant potency threshold (HRPT) for ERα agonism
- PMID: 29632997
- PMCID: PMC5962616
- DOI: 10.1007/s00204-018-2186-z
Human-relevant potency threshold (HRPT) for ERα agonism
Abstract
The European Commission has recently proposed draft criteria for the identification of endocrine disrupting chemicals (EDCs) that pose a significant hazard to humans or the environment. Identifying and characterizing toxic hazards based on the manner by which adverse effects are produced rather than on the nature of those adverse effects departs from traditional practice and requires a proper interpretation of the evidence regarding the chemical's ability to produce physiological effect(s) via a specific mode of action (MoA). The ability of any chemical to produce a physiological effect depends on its pharmacokinetics and the potency by which it acts via the various MoAs that can lead to the particular effect. A chemical's potency for a specific MoA-its mechanistic potency-is determined by two properties: (1) its affinity for the functional components that comprise the MoA, i.e., its specific receptors, enzymes, transporters, transcriptional elements, etc., and (2) its ability to alter the functional state of those components (activity). Using the agonist MoA via estrogen receptor alpha, we illustrate an empirical method for determining a human-relevant potency threshold (HRPT), defined as the minimum level of mechanistic potency necessary for a chemical to be able to act via a particular MoA in humans. One important use for an HRPT is to distinguish between chemicals that may be capable of, versus those likely to be incapable of, producing adverse effects in humans via the specified MoA. The method involves comparing chemicals that have different ERα agonist potencies with the ability of those chemicals to produce ERα-mediated agonist responses in human clinical trials. Based on this approach, we propose an HRPT for ERα agonism of 1E-04 relative to the potency of the endogenous estrogenic hormone 17β-estradiol or the pharmaceutical estrogen, 17α-ethinylestradiol. This approach provides a practical way to address Hazard Identification according to the draft criteria for identification of EDCs recently proposed by the European Commission.
Keywords: Endocrine disruptor/disruption; Estrogen system; European Commission; HRPT; Hazard identification; Potency.
Figures
References
-
- Adolphe JL, Whiting SJ, Juurlink BH, Thorpe LU, Alcorn J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br J Nutr. 2010;103:929–938. - PubMed
-
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2009;29(3):730–741. - PubMed
-
- AOP Wiki (2017) https://aopwiki.org/#Wiki%202.0%20Upgrade. Accessed 13 June 2017
-
- Armstrong DT, Moon YS, Leung PC. Uterotrophic effects of testosterone and 5alpha-dihydrotestosterone in intact and ovariectomized immature female rats. Biol Reprod. 1976;15:107–114. - PubMed
-
- Barlas N, Özer S, Karabulut G. The estrogenic effects of apigenin, phloretin and myricetin based on uterotrophic assay in immature Wistar albino rats. Toxicol Lett. 2014;226:35–42. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous