Solid nuclei and liquid droplets: A parallel treatment for 3 phase systems
- PMID: 29633411
- PMCID: PMC6032351
- DOI: 10.1002/pro.3419
Solid nuclei and liquid droplets: A parallel treatment for 3 phase systems
Abstract
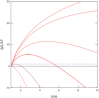
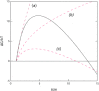
For solid phase self assembly into crystals or large diameter polymers, the presence of a liquid-liquid demixing transition has been known to have an accelerating effect on the nucleation process. We present a novel approach to the description of accelerated nucleation in which the formation of solid phase aggregates and liquid-like aggregates compete as parallel pathways to formation of dense phases. The central idea is that the small aggregates that would ultimately form the liquid phase are sufficiently labile to sample the configurations that would form the solid, so that the growing cluster begins as a liquid, and switches into growth as a solid when the aggregates have equal free energies. This can accelerate the reaction even when the liquid-demixed state is thermodynamically unfavorable. The rate-limiting barrier is therefore the energy at which there is a transition between liquid and solid, and the effective nucleus size is then concentration independent, even though for both nucleated demixing and nucleated crystallization, the nucleus size does depend on concentration. These ideas can be expressed in a chemical potential formalism that has been successfully used in nucleation of sickle hemoglobin, but not to our knowledge previously employed in describing LLD processes. The method is illustrated by considering existing data on Lysozyme.
Keywords: kinetics; liquid-liquid demixing; nucleation; protein assembly.
© 2018 The Protein Society.
Figures


Similar articles
-
Nucleation of ordered solid phases of proteins via a disordered high-density state: phenomenological approach.J Chem Phys. 2005 May 1;122(17):174905. doi: 10.1063/1.1887168. J Chem Phys. 2005. PMID: 15910067
-
Unraveling the coupling between demixing and crystallization in mixtures.J Am Chem Soc. 2014 Jun 11;136(23):8145-8. doi: 10.1021/ja500621m. Epub 2014 Feb 28. J Am Chem Soc. 2014. PMID: 24568167
-
An aggregation-volume-bias Monte Carlo investigation on the condensation of a Lennard-Jones vapor below the triple point and crystal nucleation in cluster systems: an in-depth evaluation of the classical nucleation theory.J Phys Chem B. 2008 Apr 3;112(13):4067-78. doi: 10.1021/jp709693g. Epub 2008 Mar 12. J Phys Chem B. 2008. PMID: 18335920
-
Nonclassical nucleation pathways in protein crystallization.J Phys Condens Matter. 2017 Nov 8;29(44):443002. doi: 10.1088/1361-648X/aa8253. J Phys Condens Matter. 2017. PMID: 28984274 Review.
-
Recent developments in the kinetic theory of nucleation.Adv Colloid Interface Sci. 2005 Dec 30;118(1-3):51-72. doi: 10.1016/j.cis.2005.06.001. Epub 2005 Aug 30. Adv Colloid Interface Sci. 2005. PMID: 16137628 Review.
References
-
- Gunton JD, Shiryayev A, Pagan DL (2007) Protein Condensation. Cambridge: Cambridge University Press.
-
- Becker R, Döring W (1935) Kinetische Behandlung der Keimbildung in übersättigten Dämpfen. Ann Physik 416:719–752.
-
- ten Wolde PR, Frenkel D (1997) Enhancement of protein crystal nucleation by critical density fluctuations. Science 277:1975–1978. - PubMed
-
- Pan W, Kolomeisky AB, Vekilov PG (2005) Nucleation of ordered solid phases of proteins via a disordered high‐density state: phenomenological approach. J Chem Phys 122:174905. - PubMed
-
- Ivanova M, Jasuja R, Krasnosselskaia L, Josephs R, Wang Z, Ding M, Horiuchi K, Adachi K, Ferrone FA (2001) Flexibility and nucleation in sickle hemoglobin. J Mol Biol 314:851–861. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

