Pharmacological differences and clinical implications of various botulinum toxin preparations: a critical appraisal
- PMID: 29633692
- PMCID: PMC5901944
- DOI: 10.11138/fneur/2018.33.1.007
Pharmacological differences and clinical implications of various botulinum toxin preparations: a critical appraisal
Abstract
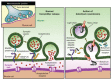
Three different type A botulinum neurotoxins (BoNTAs) - onabotulinumtoxinA, abobotulinumtoxinA and incobotulinumtoxinA) - are currently marketed in Europe to treat several conditions. Differences between BoNTA preparations, which depend on their specific biotypes and manufacturing processes, lead to clinically relevant pharmacotherapeutic dissimilarities. All three available products are separately recognized and reviewed in American Academy of Neurology guidelines. The neurotoxin load/100U is likewise different among the different BoNTAs, with the result that the specific potency of the 150kD BoNTA neurotoxin is calculated as 137 units/ng for onabotulinumtoxinA, 154 units/ng for abobotulinumtoxinA, and 227 units/ng for incobotulinumtoxinA. It is important for clinicians to have all three BoNTAs available in order to choose the most suitable preparation for the specific indication in the single patient. Commercially available BoNTAs must be recognized as different from one another, and therefore as non-interchangeable. The essential experience of the clinician is of the utmost importance in choosing the most appropriate treatment.
Figures
References
-
- Alam M. Practice gaps. Dilution, reconstitution, and complexity: comment on “A small study of the relationship between abobotulinum toxin A concentration and forehead wrinkle reduction”. Arch Dermatol. 2012;148:121–122. - PubMed
-
- Albanese A. Terminology for preparations of botulinum neurotoxins: what a difference a name makes. JAMA. 2011;305:89–90. - PubMed
-
- Aoki KR. A comparison of the safety margins of botulinum neurotoxin serotypes A, B, and F in mice. Toxicon. 2001;39:1815–1820. - PubMed
-
- Aoki KR. Botulinum neurotoxin serotypes A and B preparations have different safety margins in preclinical models of muscle weakening efficacy and systemic safety. Toxicon. 2002;40:923–928. - PubMed
-
- Aoki K, Sartorius A, Ardila C, et al. Pharmacology of Botox®, Dysport®, Myobloc and BOTOX-A in animal models of efficacy and safety. 5th International Conference on Basic and Therapeutic Aspects of Botulinum and Tetanus Toxins; Denver, CO. 2005; 2005. p. 59.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical