Dynamic representation of 3D auditory space in the midbrain of the free-flying echolocating bat
- PMID: 29633711
- PMCID: PMC5896882
- DOI: 10.7554/eLife.29053
Dynamic representation of 3D auditory space in the midbrain of the free-flying echolocating bat
Abstract
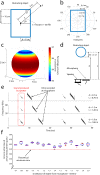
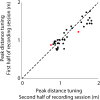
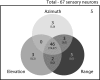
Essential to spatial orientation in the natural environment is a dynamic representation of direction and distance to objects. Despite the importance of 3D spatial localization to parse objects in the environment and to guide movement, most neurophysiological investigations of sensory mapping have been limited to studies of restrained subjects, tested with 2D, artificial stimuli. Here, we show for the first time that sensory neurons in the midbrain superior colliculus (SC) of the free-flying echolocating bat encode 3D egocentric space, and that the bat's inspection of objects in the physical environment sharpens tuning of single neurons, and shifts peak responses to represent closer distances. These findings emerged from wireless neural recordings in free-flying bats, in combination with an echo model that computes the animal's instantaneous stimulus space. Our research reveals dynamic 3D space coding in a freely moving mammal engaged in a real-world navigation task.
Keywords: 3D receptive fields; Eptesicus fuscus; echolocating bats; free flying; natural behavior; neuroscience; superior colliculus.
© 2018, Kothari et al.
Conflict of interest statement
NK, MW, CM No competing interests declared
Figures
















Comment in
-
Smart bats click twice.Elife. 2018 Apr 13;7:e36561. doi: 10.7554/eLife.36561. Elife. 2018. PMID: 29651985 Free PMC article.
Similar articles
-
Functional Organization and Dynamic Activity in the Superior Colliculus of the Echolocating Bat, Eptesicus fuscus.J Neurosci. 2018 Jan 3;38(1):245-256. doi: 10.1523/JNEUROSCI.1775-17.2017. Epub 2017 Nov 27. J Neurosci. 2018. PMID: 29180610 Free PMC article.
-
Natural echolocation sequences evoke echo-delay selectivity in the auditory midbrain of the FM bat, Eptesicus fuscus.J Neurophysiol. 2018 Sep 1;120(3):1323-1339. doi: 10.1152/jn.00160.2018. Epub 2018 Jun 20. J Neurophysiol. 2018. PMID: 29924708
-
Orienting responses and vocalizations produced by microstimulation in the superior colliculus of the echolocating bat, Eptesicus fuscus.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002 Mar;188(2):89-108. doi: 10.1007/s00359-001-0275-5. Epub 2002 Feb 27. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002. PMID: 11919691
-
Representation of perceptual dimensions of insect prey during terminal pursuit by echolocating bats.Biol Bull. 1996 Aug;191(1):109-21. doi: 10.2307/1543071. Biol Bull. 1996. PMID: 8776847 Review.
-
A view of the world through the bat's ear: the formation of acoustic images in echolocation.Cognition. 1989 Nov;33(1-2):155-99. doi: 10.1016/0010-0277(89)90009-7. Cognition. 1989. PMID: 2691182 Review.
Cited by
-
3D Hippocampal Place Field Dynamics in Free-Flying Echolocating Bats.Front Cell Neurosci. 2018 Aug 23;12:270. doi: 10.3389/fncel.2018.00270. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30190673 Free PMC article.
-
Spatial attention in natural tasks [version 1; peer review: 2 approved with reservations].Mol Psychol. 2022;1:4. doi: 10.12688/molpsychol.17488.1. Epub 2022 Dec 22. Mol Psychol. 2022. PMID: 37325441 Free PMC article.
-
Are Grid-Like Representations a Component of All Perception and Cognition?Front Neural Circuits. 2022 Jul 14;16:924016. doi: 10.3389/fncir.2022.924016. eCollection 2022. Front Neural Circuits. 2022. PMID: 35911570 Free PMC article.
-
Natural acoustic stimuli evoke selective responses in the hippocampus of passive listening bats.Hippocampus. 2022 Apr;32(4):298-309. doi: 10.1002/hipo.23407. Epub 2022 Jan 27. Hippocampus. 2022. PMID: 35085416 Free PMC article.
-
Smart bats click twice.Elife. 2018 Apr 13;7:e36561. doi: 10.7554/eLife.36561. Elife. 2018. PMID: 29651985 Free PMC article.
References
-
- Altmann S. Rotations, Quaternions, and Double Groups. Mineola: Courier Corporation; 2005.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

